



版权说明:本文档由用户提供并上传,收益归属内容提供方,若内容存在侵权,请进行举报或认领
文档简介
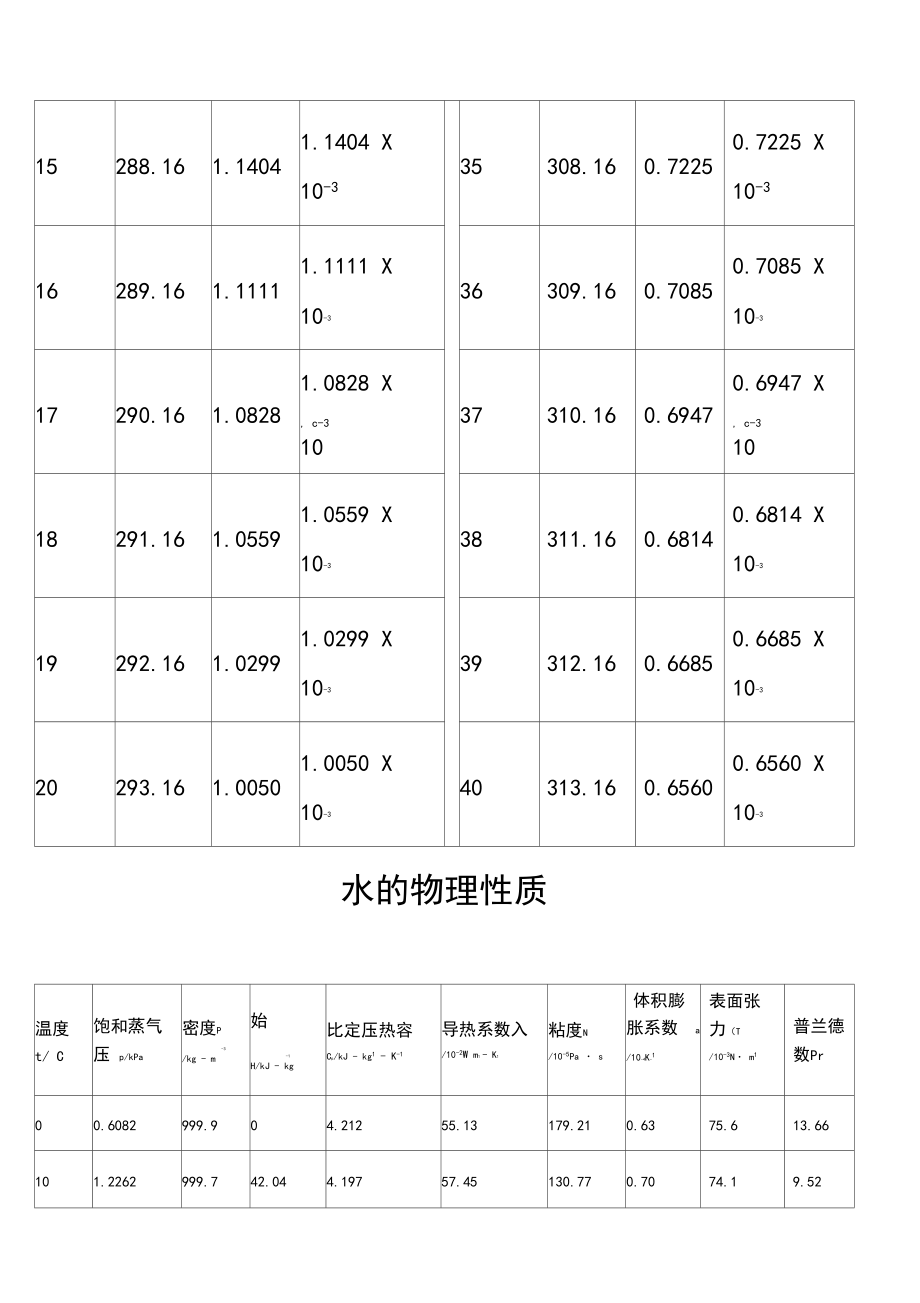
1、水的黏度表(040C)温度T粘度1Pa , s 或N s , m-2温度T粘度Pa , s 或n s mCKcK0273.161.79211.7921 X10-320.2293.361.00001.0000 x10-31274.161.73131.7313 X10-321294.160.98100.9810 x10-32275.161.67281.6728 X10-322295.160.95790.9579 x10-33276.161.61911.6191 X, c-31023296.160.93580.9358 x, c-3104277.161.56741.5674 X10-324297.1
2、60.91420.9142 X10-35278.161.51881.5188 X10-325298.160.89370.8937 X10-36279.161.47281.4728 X26299.160.87370.8737 X, c-310, c-3107280.161.42841.4284 X10-327300.160.85450.8545 X10-38281.161.38601.3860 X10-328301.160.83600.8360 X10-39282.161.34621.3462 X10-329302.160.81800.8180 X10-310283.161.30771.3077
3、 X10-330303.160.80070.8007 X10-311284.161.27131.2713 X, c-31031304.160.78400.7840 X, c-31012285.161.23631.2363 X10-332305.160.76790.7679 X10-313286.161.20281.2028 X10-333306.160.75230.7523 X10-314287.161.17091.1709 X10-334307.160.73710.7371 X10-315288.161.14041.1404 X10-335308.160.72250.7225 X10-316
4、289.161.11111.1111 X10-336309.160.70850.7085 X10-317290.161.08281.0828 X, c-31037310.160.69470.6947 X, c-31018291.161.05591.0559 X10-338311.160.68140.6814 X10-319292.161.02991.0299 X10-339312.160.66850.6685 X10-320293.161.00501.0050 X10-340313.160.65600.6560 X10-3水的物理性质温度 t/ C饱和蒸气压 p/kPa密度P-3/kg - m
5、始-1H/kJ - kg比定压热容Cp/kJ - kg1 - K-1导热系数入 /10-2W m1 - K1粘度N/10-5Pa s体积膨 胀系数 a /10-4K-1表面张 力(T/10-3N m1普兰德数Pr00.6082999.904.21255.13179.210.6375.613.66101.2262999.742.044.19757.45130.770.7074.19.52202.3346998.283.904.18359.89100.501.8272.67.01304.2474995.7125.694.17461.7680.073.2171.25.42407.3766992.21
6、65.714.17463.3865.603.8769.64.325012.31988.1209.304.17464.7854.944.4967.73.546019.932983.2251.124.17865.9446.885.1166.22.987031.164977.8292.994.17866.7640.615.7064.32.548047.379971.8334.944.19567.4535.656.3262.62.229070.136965.3376.984.20867.9831.656.9560.71.96100101.33958.4419.104.22068.0428.387.52
7、58.81.76110143.31951.0461.344.23868.2725.898.0856.91.61120198.64943.1503.674.25068.5023.738.6454.81.47130270.25934.8546.384.26668.5021.779.1752.81.36140361.47926.1589.084.28768.2720.109.7250.71.26150476.24917.0632.204.31268.3818.6310.348.61.18160618.28907.4675.334.34668.2717.3610.746.61.11170792.598
8、97.3719.294.37967.9216.2811.345.31.051801003.5886.9763.254.41767.4515.3011.942.31.001901255.6876.0807.634.46066.9914.4212.640.80.962001554.77863.0852.434.50566.2913.6313.338.40.932101917.72852.8897.654.55565.4813.0414.136.10.912202320.88840.3943.704.61464.5512.4614.833.80.892302798.59827.3990.184.68
9、163.7311.9715.931.60.882403347.91813.61037.494.75662.8011.4716.829.10.872503977.67799.01085.644.84461.7610.9818.126.70.862604693.75784.01135.044.94960.8410.5919.724.20.872705503.99767.91185.285.07059.9610.2021.621.90.882806417.24750.71236.285.22957.459.8123.719.50.892907443.29732.31289.955.48555.829
10、.4226.217.20.933008592.94712.51344.805.73653.969.1229.214.70.973109877.96691.11402.166.07152.348.8332.912.31.0232011300.3667.11462.036.57350.598.5338.210.01.1133012879.6640.21526.197.24348.738.1443.37.821.2234014615.9610.11594.758.16445.717.7553.45.781.3835016538.5574.41671.379.50443.037.2666.83.891
11、.6036018667.1528.01761.3913.98439.546.671092.062.3637021040.9450.51892.4340.31933.735.692640.486.80C)F3? Viscosity decreases with pressure(at temperatures below 33Viscous flow occurs by molecules moving through the voids that exist between them. As the pressure increases, the volume decreases and th
12、e volume of these voids reduces, so normallyincreasing pressure increases the viscosity.Water's pressure-viscosity behavior 534 can be explained by the increased pressure (up to about 150 MPa) causing deformation, so reducing the strength of the hydrogen-bonded network, which is also partially r
13、esponsible for the viscosity. This reduction in cohesivity more than compensates for the reduced void volume. It is thus a direct consequence of the balance between hydrogen bonding effects and the van der Waals dispersion forces 558 in water; hydrogen bonding prevailing at lower temperatures and pr
14、essures. At higher pressures (and densities), the balance between hydrogen bonding effects and the van der Waals dispersion forces is tipped in favor of the dispersion forces and the remaining hydrogen bonds are stronger due to the closer proximity of the contributing oxygen atoms 655. Viscosity, then, increases with pressure. The dashed line (opposite) indicates the viscosity minima.The variation of viscosity with pressure and temperature has been used as evidence that the viscosity is determined more by the extent
温馨提示
- 1. 本站所有资源如无特殊说明,都需要本地电脑安装OFFICE2007和PDF阅读器。图纸软件为CAD,CAXA,PROE,UG,SolidWorks等.压缩文件请下载最新的WinRAR软件解压。
- 2. 本站的文档不包含任何第三方提供的附件图纸等,如果需要附件,请联系上传者。文件的所有权益归上传用户所有。
- 3. 本站RAR压缩包中若带图纸,网页内容里面会有图纸预览,若没有图纸预览就没有图纸。
- 4. 未经权益所有人同意不得将文件中的内容挪作商业或盈利用途。
- 5. 人人文库网仅提供信息存储空间,仅对用户上传内容的表现方式做保护处理,对用户上传分享的文档内容本身不做任何修改或编辑,并不能对任何下载内容负责。
- 6. 下载文件中如有侵权或不适当内容,请与我们联系,我们立即纠正。
- 7. 本站不保证下载资源的准确性、安全性和完整性, 同时也不承担用户因使用这些下载资源对自己和他人造成任何形式的伤害或损失。
最新文档
- 留学行李寄存与存放补充协议
- 货币市场基金资金来源补充协议
- 高档家具跨国运输全程保险合同
- 智能制造车间技术升级补充协议
- 创新调解离婚子女临时探视应急协议
- 商业地产项目投资合作与风险控制协议
- 公共场所智能灯光控制系统设计、安装与维护合同
- 悬疑推理小说改编影视作品授权合同
- 互联网金融服务交易风险防控补充协议
- 医院培训课件:《导管相关血流感染管理要求》
- 颈椎病课件完整版
- 《松材线虫病》课件
- 《中小学校岗位安全工作指导手册》
- 《大气污染物综合排放标准》编制说明
- 养老机构入住潜在风险告知书1-3-5
- 北京四中2025届高一物理第一学期期中经典试题含解析
- 《剪映专业版:短视频创作案例教程(全彩慕课版)》 课件 第5章 创作城市宣传片
- 企业名称:个人防护用品(PPE)管理规定
- 深圳市业主共有资金监督管理办法
- 接力版六年级下册小学英语全册同步练习(一课一练)
- 雾化吸入疗法合理用药专家共识(2024版)解读

评论
0/150
提交评论