



版权说明:本文档由用户提供并上传,收益归属内容提供方,若内容存在侵权,请进行举报或认领
文档简介
1、整理pptA reaction between two aromatic aldehydes catalyzed by cyanide anion to give benzoins (-hydroxyketones)5) Benzoin CondensationHOOOHNaCNbenzoin(-hydroxyketone)PhHCO+CN-PhCOHCN-PhCCNOH-HPhCOPhCHOCOHCNPh-PhCHOHCOCNPh-PhOPhOHMechanism:整理pptThe uniquely successful role of cyanide ion in catalyzing b
2、enzoin reaction is due to its four qualities: (i) high nucleophilic activity (ii) facilitating the proton transfer (iii) ability to stabilize negative charge in active aldehyde intermediate (iv) ability to depart finallyNature has been performing this task efficiently in acompletely analogous manner
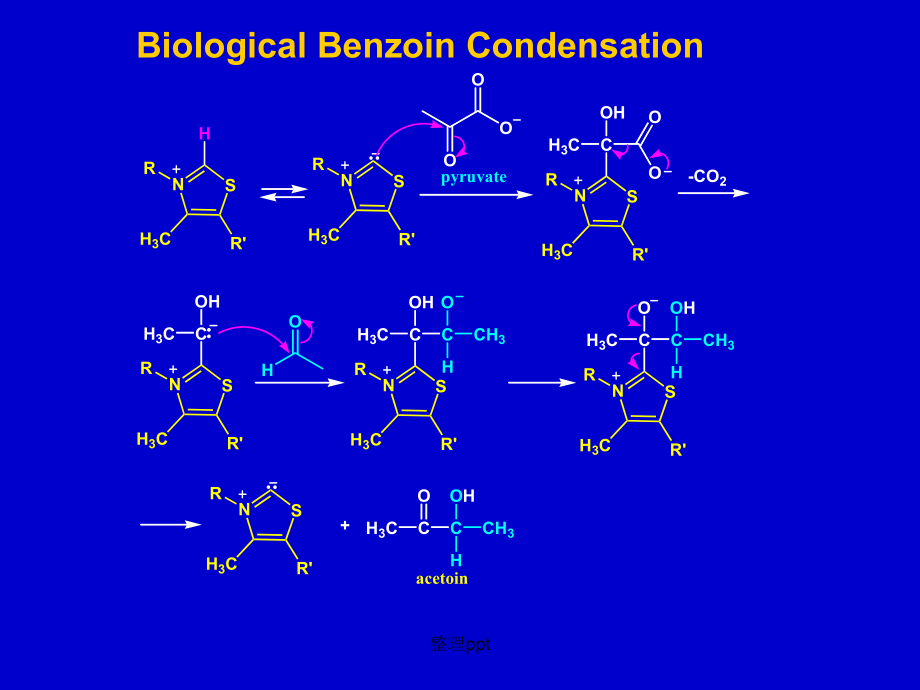
3、 using (vitamin B1) thiamine pyrophosphate,TPP, a coenzyme present in our body, and other living organisms.NNNNH2H3CSH3COHVitamine B1 (Thiamine)NNNNH2H3CSH3COPOOOPOOOThiamine pyrophosphate, TPP整理pptNRSH3CRHNRSH3CROOOpyruvateNRSH3CRCH3COHOO-CO2NRSH3CRCH3COHHONRSH3CRCH3COHCCH3OHNRSH3CRCH3COCCH3OHHNRSH
4、3CRH3CCOCOHCH3H+acetoinBiological Benzoin Condensation整理pptAddition of Carbon Nucleophiles in the Synthesis of DrugsOCCMgBrOHHBrCH2NHCH3NCH3BrTerbinafine整理pptHCCMgBrO -InononeCCHOHH2LindlarcatalystOHHClPh3PPPh3+Cl-OAcNaOCH3OAcOVitamin A acetate整理ppt3.1.2. Addition of Oxygen Nucleophiles1) Addition o
5、f Water (Hydration): Aldehydes and ketones react with water to yield 1,1-diols (geminal diols, or carbonyl hydrates) Hyrdation is reversibleRRCO+H2ORRCHOOHaldehyde or ketonegeminal diol (hydrate)KCl3CCHO K 104 HCHO K 103 CH3CCHO K 1CH3COCH3 K 10-2整理pptCOMechanism of Base-Catalyzed Hydration:+OHCOOHh
6、ydroxy alkoxideHOHCOHOHOH+geminal diolMechanism of Acid-Catalyzed Hydration:+COH+ +COH HOHCOH+OHHCOHOH+geminal diolH+protonationNucleophilic attackNucleophilic attackproton transferdeprotonation整理ppt (a) Rank in order of increasing favorability of hydration: Cl3CCHO, Cl3CCOCH3, Cl3CCOCCl3.(b) Treatm
7、ent of acetone with H218O results in the formation of labeled acetone, CH3CO18CH3. Explain.整理ppt2) Addition of Alcohol: Alcohols are weak nucleophiles but acid promotes addition Hemiacetals are very unstable The position of equilibrium is favorable for acetal formation from most aldehydes For most k
8、etones, the position of equilibrium is unfavorableRHCOROH, H+aldehydeRCOHHORhemiacetalROH, H+RCORHOR+ H2OacetalRRCOROH, H+RCOHRORketonehemiketalROH, H+RCORRORketal+ H2O整理ppt An aldehyde (or ketone) in the presence of excess alcohol and an acid catalyst will form an acetall Formation of the acetal pr
9、oceeds via the corresponding hemiacetall An acetal has two alkoxyl groups bonded to the same carbon Acetals are stable when isolated and purified Acetal formation is reversiblel An excess of water in the presence of an acid catalyst will hydrolyze an acetal to the corresponding aldehyde (or ketone)整
10、理pptMechanism of Acetal Formation:+COH+ +COH protonationNucleophilic attackdeprotonationHORCOH+ORHCOHORH+COH2OR+CORHORCORORH+protonationloss of waterNucleophilic attackdeprotonationCORORAcetalH+整理pptCyclic Acetals Acetal formation from ketones and simple alcohols is less favorable than formation fro
11、m aldehydes Formation of cyclic 5- and 6- membered ring acetals from ketones is more easy to formRRCO H+ketoneHOCH2CH2CH2OH+RR+ H2Ocyclic acetalOO H+RRCOketoneHOCH2CH2OH+RRCOO+H2Ocyclic acetalDean-Stark trap整理ppt Acetals tolerate bases, oxidizing and reducing agents, and organometallic reagents, ect
12、. but are sensitive to acids An excess of water in the presence of an acid catalyst will hydrolyze an acetal to the corresponding aldehyde (or ketone)RRCO H+aldehydeorketone+RRCORRO+H2O2 ROH(excess)整理pptUses of Acetals: as Protection groups HO+BrHO?OHHOBrHOOHOHHOH+,BrOOMgBrMgOOHOOOOHHOH2O, H+整理pptOH
13、?OHHOOHOHOHHOH+,OOH2O, H+KMnO4 (dilut.)OOHOOHHOHOOHProtection of Hydroxy Group:OROH+H+ROOTetrahydropyranyletherDihydropyran整理ppt Cyclic hemiacetals are formed by intramolecular cyclization of hydroxy aldehydes Cyclic Hemiacetals containing five- and six-membered rings are stable compoundsCyclic Hemi
14、acetals=OHHOchiral *COOH6%94%HOHO5-hydroxypentanalHOHO=5-hydroxybutanalOHHOOOH11%89%chiral *C整理ppt3.1.3. Addition of Sulfur Nucleophiles1) Addition of Sodium Bisulfite Sodium bisulfite adds to aldehydes and unhindered ketones (aliphatic methyl ketones and 99%)(1.5 X 10-4%)CyclohexanoneOOH(98.8%)(1.2
15、%)整理ppt-Dicarbonyl compounds exist primarily in the enol formOOOHOPentane-2,4-dione(24%)Enol form(76%)OOHOOH+-Hydrogen bondHydrogen bondResonance stabilization of the enol form The greater stability of the enol form of -dicarbonyl compounds can be attributed to resonance stabilization of the conjuga
16、ted double bonds and (in a cyclic form) through hydrogen bonding整理pptMechanism of Base-Catalyzed EnolizationCHCOHO-CCO-Enolate(achiral)HOHCCOHEnol(achiral)HO-+Mechanism of Acid-Catalyzed EnolizationCHCOHOHH+CHCO+HOHH+CCOHEnol(achiral)HOH2+整理pptKeto-Enol TautomerismlCarbonyl compounds with hydrogens
17、bonded to their carbons equilibrate with their corresponding enols. lThis rapid equilibration is called tautomerism, and the individual isomers are tautomers.lUnlike resonance forms, tautomers are isomers.lDespite the fact that very little of the enol isomer is present at room temperature, enols are
18、 very important because they are reactive. 整理ppt The pKas of most simple aldehydes and ketones are about 1620 Both the carbonyl compound and its enolate are present when an acidbase equilibrium is established with hydroxide ionFormation and Stability of EnolateHO-CHCCH3OH3CHOH+CHHCH3COH3C-2-Methylpr
19、opanalpKa = 15.5Enolate of 2-methylpropanal+WaterpKa = 15.7K = 1整理ppt Aldehydes and ketones can be converted completely to their enolates by using very strong bases such as lithium diisopropylamide (CH3)2CH2NLi (LDA)-CPhCHOH+CHPhCHOH-AcetophenonepKa = 18.3Enolate of acetophenone+pKa = 36K = 1018(H3C
20、)2HCN(H3C)2HCDiisopropylamide ion(H3C)2HCNH(H3C)2HCDiisopropylamine整理ppt Kinetic Control. A less crowded proton is removed faster than a more crowded one to form less stable enolate (kinetic enolate) Thermodynamic Control. A more crowded proton is removed to form more stable enolate with the more su
21、bstituted double bond (thermodynamic enolate)Enolate RegiochemistryOCH3HHHEnolate can be formedby removing either ofthese two hydrogens26basesolventtemperature-OCH3HHKinetic enolate-basesolventtemperatureOCH3HHThermodynamic enolate整理pptThe kinetic enolate is favored by: A strong nonnucleophilic base
22、. LDA is a strong, sterically hindered base Polar aprotic solvent. THF is both polar and aprotic Low temperature (-78 )The thermodynamic enolate is favored by: A strong base. Na+ OCH2CH3, K+ OC(CH3)3, or other alkoxides are common Protic solvent. CH3CH2OH or other alcohols. Room temperature (25 ).整理
23、ppt Enolates is an ambident nucleophileReactions of Enols and Enolates:HCCOCCOHCCOH+-E+CCEOH+new bond on the carbonHCCOB- CCO-Enols:Enolates:CCO-two reactive sitesE+-H+CCEO CCEO new bondE+CCOEnew bondpreferred pathwayThis path does notusually occur整理ppt3.2.2. The Aldol CondensationIn the presence of
24、 acid or base, two aldehydes (or ketones) react each other to form a -hydroxy aldehyde (or -hydroxy ketone)(aldol from aldehyde and alcohol)Aldol addition or Aldol reactionCHCRHHOCHCRHHO+CCCRHHOHHCHOHRH+ or OH- Hydroxy aldehyde(aldol product)CCCRHHHCHOR unsaturated aldehydeUnder acidic conditions, t
25、he reaction generally leads directly to the dehydration product整理pptMechanism of Base-Catalyzed Aldol CondensationHHCCOHB-HHCCO-HHCCO-HHCCOHHHCCOCCOHHH- HBHHCCOCCOHHHHB-B-HCCOCCOHHHH- HB- OH-HCCOCCHHH-hydroxy aldehyde,-unsaturated aldehydeNucleophilic attackproton transferproton transferproton trans
26、ferelimination The products of aldol addition undergo dehydration on heating, to yield , -unsaturated aldehydes整理pptMechanism of Acid-Catalyzed Aldol Condensation This reaction generally leads directly to the dehydration productHHCCOHHHCCOHHH+HHCCOH- H+HHCCOHH+HHCCOHCCOHHHH+HCCOCHCOH2HHH+- OH2HCCOCC
27、HHH,-unsaturated aldehydeNucleophilic attackproton transfereliminationprotonationdeprotonation整理pptSynthetic ApplicationsRCH2CHO2OH-AldehydeRCH2CHCHCHOHROAdolNaBH4RCH2CHCHCH2OHOHR1,3-DiolH+orheatingRCH2CHCCHRO Unsaturated aldehydeLiAlH4RCH2CHCCH2OHRAllylic alcoholH2/NihighpressureRCH2CH2CHCH2OHRSatu
28、rated alcoholRCH2CH2CHCHROH2, Pd-CAldehyde1,4-AdditionRCH2CHCHCHNuRO整理pptCrossed Aldol Reactions Crossed aldol reactions (aldol reactions involving two different aldehydes) are of little use when they lead to a mixture of productsRCH2CHOOH-+RCH2CHOH2ORCH2CHCHCHOHRORCH2CHCHCH+OHRORCH2CHCHCH+OROHRCH2C
29、HCHCHOHRO+整理pptPractical Crossed Aldol Reactions Crossed aldol reactions give one predictable product when one of the reaction partners has no -hydrogen整理pptCyclization via Aldol CondensationIntramolecular reaction of dicarbonyl compounds proceeds to form five- and six-membered rings preferentiallyO
30、H-OHOOOH-HOOORRORHOKetones areless reactive towardnucleophilesAledehydes aremore reactive towardnucleophiles整理pptWhy Meat from Younger Animals Is Softer? One of the most abundant proteins found in mammals is called collagen. Individual molecules of collagen can be isolated from young animals, but no
31、t from older animals, because with age, collagen molecules cross-link with each other via an aldol condensation reaction. First, amino groups located on side chains of collagen are converted to aldehyde groups via a process called oxidative deamination. Then, the resulting aldehyde groups can underg
32、o an aldol condensation. This process results in the cross-linking of two collagen molecules. As an animal ages, the number of cross-linked proteins increases at the expense of the individual collagen molecules.整理ppt A Biological Aldol Condensation整理ppt3.2.3. Halogenation at the Carbon Carbonyl comp
33、ounds bearing an a hydrogen can be halogenated at the a carbon in the presence of acid or base.CCHO+X2H+ or OH-CCXO整理pptAcid-catalyzed halogenationAcid-catalyzed halogenation proceeds via the enolAcid generated by the reaction catalyzes further reaction (autocatalytic)Acid-catalyzed halogenation can
34、 halogenate only one or two -HsHalogenation slows down enolizationXXCCXOH+X-+CCXOHX+CCXOElectronwithdrawingless basic thanunsaturated ketoneCCXOH+- H+CCOHHCCO H+整理pptBase-Promoted Halogenation Base-promoted halogenation occurs via an enolate Base is consumed The -halo ketone produced is more reactiv
35、e than ketone Enolate ion stabilized by e-withdrawing halogenHCCOOH-H2OCCO-CCO-XXCCXOX-+CCHXOOH-H2OXCCO-CCOX-enolate stablized by X整理pptMultiple HalogenationOH-H2OHCCOHHCCHHXO-X-OH-H2OCCOHH-XXCCOHX-XX-X-CCXHXOOH-H2OCCOXX-XX-X-CCXXXO整理ppt3.2.4. Haloform ReactionA methyl ketone is treated with excess
36、base and excess halogen to afford a carboxylic acid after acidic workupRCH3OX2, NaOHRO-OH+ROHORCH3OX2, NaOHRCX3OOH-RCX3OOHMechanism:+-ROHOCX3+-ROOHCX3HaloformCarboxylateanion整理pptUses of Haloform Reaction1) Converting methyl ketones to carboxylic acidsCH3CC(CH3)3O+1) Br2, NaOH, H2O2) H3O+(CH3)3CCO2H
37、CHBr32,2-dimethybutanone2,2-dimethypropanoicacidbromoform Chlorine and bromine are most commonly used as the halogen component for the purposeCOCH31) NaOCl, H2O2) H3O+COOH+CHCl3bromoform1-cyclopropylethanonecyclopropanoic acid整理ppt2) The iodoform classification test(H)RCH3COI2, NaOH, H2O(H)RCOONa +C
38、HI3methyl ketones(acetaldehyde)idoform(H)RCH3CHOX2, NaOH(H)RCH3COI2, NaOH, H2OHmethyl ketones(acetaldehyde)methyl 2 alcohols(ethanol)(H)RCOONa +CHI3idoforml The iodoform reaction is used in the classification test for methyl ketones and methyl secondary alcohols整理pptCH3OCH3OOHONaHSO3 (40%)NO REACTIO
39、NI2, NaOHCHI3No reactionNo reaction?整理pptChloroform in Tap WaterWhen water is chlorinated to purify it for public consumption, chloroform is produced from organic impurities in the water via the haloform reaction. (Many of these organic impurities are naturally occurring, such as humic substances.)
40、The presence of chloroform in public water is of concern for water treatment plants and environmental officers, because chloroform is carcinogenic. Thus, the technology that solves one problem creates another. It is worth recalling, however,that before chlorination of water was introduced, thousands
41、 of people died in epidemics of diseases such as cholera and dysentery.整理ppt3.2.5. Mannich ReactionCondensation of a primary or secondary amine or ammonia (usually as the hydrochloride) with formaldehyde and a compound containing at least one active hydrogen atomCarl Ulrich Franz Mannich (*March 8,
42、1877 - March 5, 1947) was a German Chemist.The Mannich reaction was named after his discovery of the mechanism in 1912 R2NH+H2CO+ H3CCRR2NCH2CH2CROOaminomethyl(Aminomethylation)整理pptR2NH+H2CNR2H+H2CO- H2OH3CRCOH+CH2RCOHH2CNR2+CRCOHCNR2HHHH+-H+CRCOCNR2HHHHMechanism:Nucleophilic attackdeprotonation整理ppt+OH2CO + CH3NH2 .HClONHCH3ONHCH3CF3Fluoxetine+ H2CO +(CH3)2NH.HClOON(CH3)2HON(CH3)2OCH3Tramadol整理ppt+OH2CO +CH2NHCH3.HClCH2NCH3OCH2NCH3NatifineO15 stepsNOH3CTroponone0.75% yieldCHOCHO+CH3NH2 .HClOCO2HCO2H+1 stepNOH3CTroponone90% yield整理ppt3.3. OxidationAldehydes are much
温馨提示
- 1. 本站所有资源如无特殊说明,都需要本地电脑安装OFFICE2007和PDF阅读器。图纸软件为CAD,CAXA,PROE,UG,SolidWorks等.压缩文件请下载最新的WinRAR软件解压。
- 2. 本站的文档不包含任何第三方提供的附件图纸等,如果需要附件,请联系上传者。文件的所有权益归上传用户所有。
- 3. 本站RAR压缩包中若带图纸,网页内容里面会有图纸预览,若没有图纸预览就没有图纸。
- 4. 未经权益所有人同意不得将文件中的内容挪作商业或盈利用途。
- 5. 人人文库网仅提供信息存储空间,仅对用户上传内容的表现方式做保护处理,对用户上传分享的文档内容本身不做任何修改或编辑,并不能对任何下载内容负责。
- 6. 下载文件中如有侵权或不适当内容,请与我们联系,我们立即纠正。
- 7. 本站不保证下载资源的准确性、安全性和完整性, 同时也不承担用户因使用这些下载资源对自己和他人造成任何形式的伤害或损失。
最新文档
- 2026兴业银行兰州分行校园招聘备考题库及答案详解(名师系列)
- 2025泉州银行宁德分行招聘备考题库及答案详解(典优)
- 2025安徽马鞍山市总工会社会化工会工作者招聘12人备考题库附答案详解(达标题)
- 2026广发银行汕头分行校园招聘备考题库附答案详解(轻巧夺冠)
- 2025广西北海中共合浦县委社会工作部招聘专职化社区工作者30人备考题库含答案详解(培优a卷)
- 2025中国信托商业银行人才招聘备考题库附答案详解(达标题)
- 个性化营养方案对烧伤愈合速度的影响
- 2025年甘肃省定西市渭源县社区工作者招聘10人备考题库及答案详解(各地真题)
- 2026福建省面向厦门大学选调生选拔工作备考题库及答案详解(有一套)
- 2025蒙商银行秋季校园招聘备考题库(含答案详解)
- 2025年高职特殊教育(特殊儿童康复)试题及答案
- 梧州市总工会劳模(高技能人才)创新工作室考核评分表
- 2025年基桩静荷载试验题库及答案
- 2025药品高阻隔包装材料技术升级与临床应用分析撰文
- 2025珠海农商银行社会招聘笔试题库附答案解析
- 2025中煤西北能源化工集团招聘备考题库(104人)附答案解析
- 应急预案编制与演练 课件2
- 网红协议如何签订合同
- 2025年「焦虑情绪」内容洞察数据报告(小红书平台)
- DBJ-T13-205-2021 福建省城市地下管线信息数据库建库规程
- 抚州市总工会2025年公开招聘工会社会工作者【18人】笔试考试参考试题附答案解析

评论
0/150
提交评论