【温馨提示】 购买原稿文件请充值后自助下载。
[全部文件] 那张截图中的文件为本资料所有内容,下载后即可获得。
预览截图请勿抄袭,原稿文件完整清晰,无水印,可编辑。
有疑问可以咨询QQ:414951605或1304139763
摘要
本次毕业设计任务是完成电话机外壳的一模一腔注塑模具设计。根据塑件的结构、技术要求及企业生产的实际情况,和应用三维设计软件Pro/E进行电话机外壳的注射模设计。本设计分析塑件的工艺性,设计了塑件的注塑模具结构,并通过型腔压力和锁模力的计算,选择注塑机,给出了相关工艺参数。分别对浇注系统、分型面的选取、脱模推出机构设计、冷却结构设计、等进行了介绍。为降低模具成本,提高生产效率, 对于有国家标准的零部件,本设计均采用标准件,其中包括:注塑模标准模架;标准导柱、导套等。对于有推荐尺寸的零件,均选用推荐值设计,如浇口套等。
关键词:注射模 分型面 冷却结构 推出机构
Abstract
The graduation task is to design a complete telephone shell of a cavity-injection mold design. According to the structure of plastic parts, technical requirements and the actual situation of production, and 3-D design software Pro / E for the phone casing injection mold design. The design of plastic parts of the process, the design of plastic parts injection mold structure, and through the cavity pressure and the clamping force, the choice of injection molding machine, given the relevant technical parameters. Pouring on the respective systems, sub-surface selection, introduction agencies Stripping design, cooling structural design, etc. were introduced. To reduce die costs, increase productivity, for a national standard parts, the design of a standard parts, including: injection mold-standard mode; standards-guided, guided sets, and so on. The recommended size of the parts are optional recommended design, such as gate sets
Keywords:Injection Mould Sub-surface
Cooling structure Introduction agencies
目录
摘要 I
Abstract II
第一章 塑件的成形工艺性分析 1
1.2 ABS的注射成型工艺 1
1.3 ABS性能分析 2
1.4 ABS成型塑件的主要缺陷及消除措施: 3
第二章 注塑机的确定 4
2.1 有关塑件的计算 4
2.2 注射机型号的确定 4
2.3 注射机有关工艺参数的校核 4
第三章 分型面位置的确定 7
3.1 分型面的形式 7
3.2 分型面的设计原则 7
第四章 模型腔排列方式的确定 8
4.1 确定型腔数量及排列方式 8
第五章 注射模浇注系统的设计 9
5.1 对浇注系统进行整体设计时,一般应遵循如下基本原则: 9
5.2 主流道的设计 9
5.3 分流道的设计 10
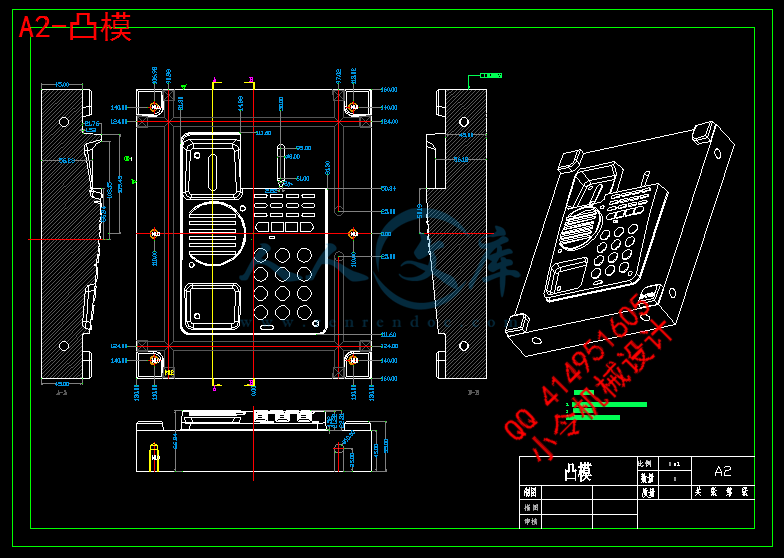
第六章 注射成型零件设计 13
6.1 成型零件的选材 13
6.2 凹模部分的结构设计 14
6.3 凸模部分的结构设计 16
第七章 脱模推出机构的设计 18
7.1 推出机构的设计要求 18
7.2 脱模阻力计算 18
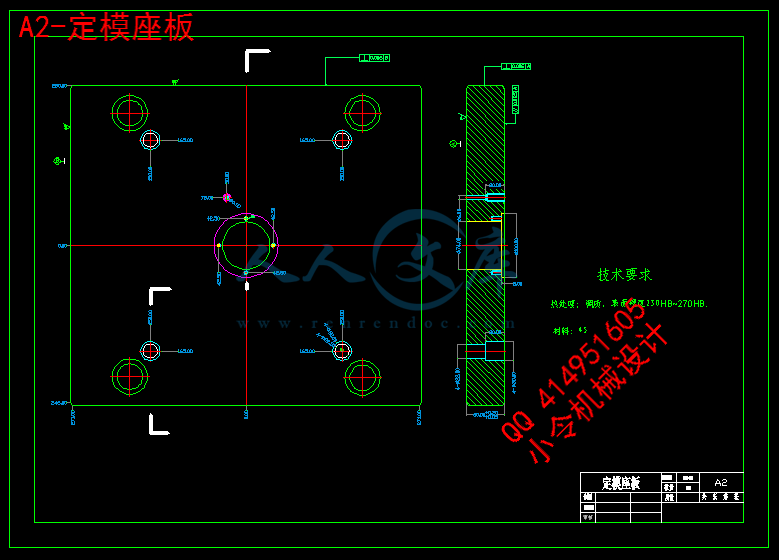
第八章 模架的确定和标准件的选用 20
8.1 定模固定板(定模座板)(500550,厚60mm) 20
8.2 定模板(500450,厚90mm) 20
8.3 动模固定板(500550,厚35mm) 20
8.4 动模板(500450,厚100mm。) 20
8.5 推杆固定板(290496,厚25mm) 20
8.6 推板(290496,厚25mm) 20
第九章 设计总结 21
致谢 22
参考文献 23
第一章 塑件的成形工艺性分析
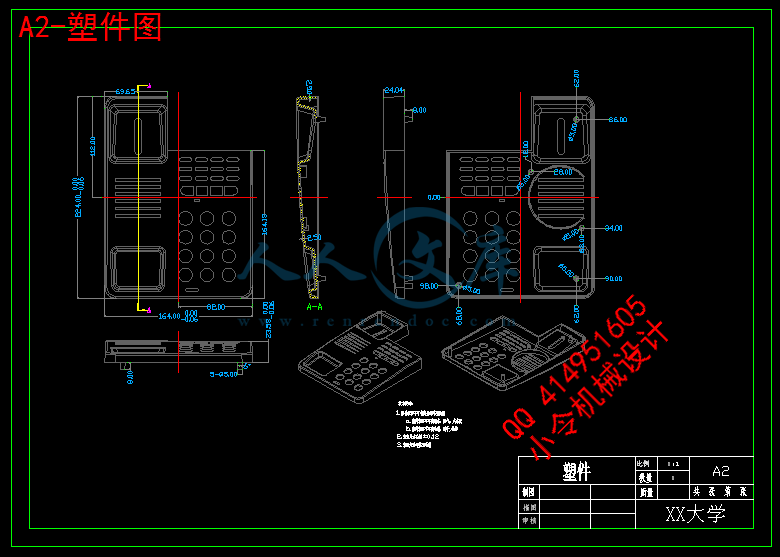
1、塑件(电话机外壳)模型图:
塑件图
2、塑件材料的选择:选用ABS(即丙烯腈-丁二烯-苯乙烯共聚物)。
3、色调:黑色。
4、生产批量:中批量。
5、塑件的结构与工艺性分析:
(1)结构分析
塑件为电话机外壳的上半部分,应有一定










 川公网安备: 51019002004831号
川公网安备: 51019002004831号