某化工仓储公司成品油及化工原料库储运工艺初步设计
收藏
资源目录

压缩包内文档预览:(预览前8页/共17页)
编号:7010214
类型:共享资源
大小:12.09MB
格式:RAR
上传时间:2018-01-06
上传人:优***
认证信息
个人认证
罗**(实名认证)
广西
IP属地:广西
40
积分
- 关 键 词:
-
化工
仓储
公司
成品油
原料库
储运
工艺
初步设计
- 资源描述:
-

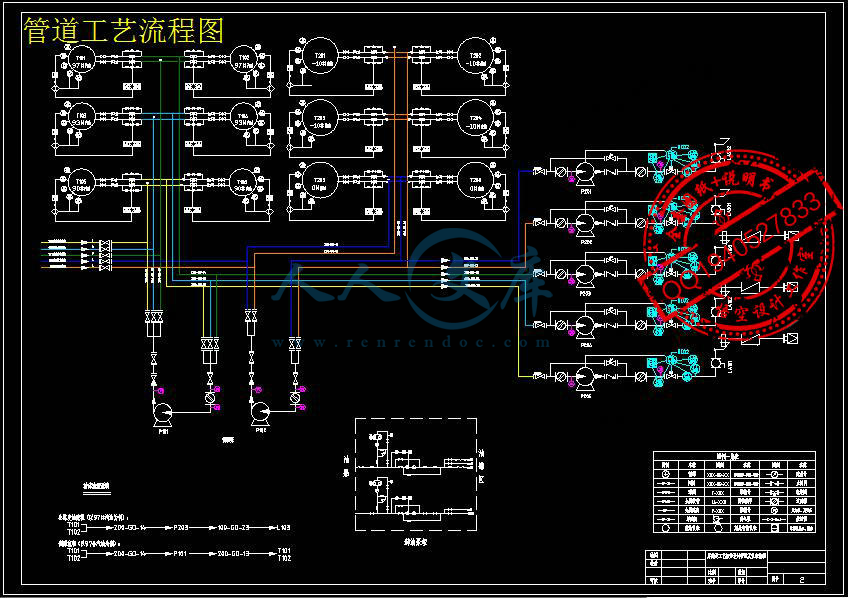






- 内容简介:
-
JOURNALOFNATURALGASCHEMISTRY212012282298REVIEWCONVENTIONALPROCESSESANDMEMBRANETECHNOLOGYFORCARBONDIOXIDEREMOVALFROMNATURALGASAREVIEWZEEYINGYEO1,THIAMLENGCHEW2,PENGWEIZHU1,ABDULRAHMANMOHAMED3,SIANGPIAOCHAI11CHEMICALENGINEERINGDISCIPLINE,SCHOOLOFENGINEERING,MONASHUNIVERSITY,46150BANDARSUNWAY,SELANGOR,MALAYSIA2FACULTYOFENGINEERINGANDGREENTECHNOLOGYFEGT,PERAKCAMPUS,UNIVERSITITUNKUABDULRAHMAN,JALANUNIVERSITI,BANDARBARAT,31900KAMPAR,PERAK,MALAYSIA3SCHOOLOFCHEMICALENGINEERING,ENGINEERINGCAMPUS,UNIVERSITISAINSMALAYSIA,SERIAMPANGAN,14300NIBONGTEBAL,PENANG,MALAYSIAMANUSCRIPTRECEIVEDJANUARY11,2012REVISEDFEBRUARY28,2012ABSTRACTMEMBRANETECHNOLOGYISBECOMINGMOREIMPORTANTFORCO2SEPARATIONFROMNATURALGASINTHENEWERADUETOITSPROCESSSIMPLICITY,RELATIVEEASEOFOPERATIONANDCONTROL,COMPACT,ANDEASYTOSCALEUPASCOMPAREDWITHCONVENTIONALPROCESSESCONVENTIONALPROCESSESSUCHASABSORPTIONANDADSORPTIONFORCO2SEPARATIONFROMNATURALGASAREGENERALLYMOREENERGYDEMANDINGANDCOSTLYFORBOTHOPERATIONANDMAINTENANCEPOLYMERICMEMBRANESARETHECURRENTCOMMERCIALMEMBRANESUSEDFORCO2SEPARATIONFROMNATURALGASHOWEVER,POLYMERICMEMBRANESPOSSESSDRAWBACKSSUCHASLOWPERMEABILITYANDSELECTIVITY,PLASTICIZATIONATHIGHTEMPERATURES,ASWELLASINSUFFICIENTTHERMALANDCHEMICALSTABILITYTHESHORTCOMINGSOFCOMMERCIALPOLYMERICMEMBRANESHAVEMOTIVATEDRESEARCHERSTOOPTFOROTHERALTERNATIVES,ESPECIALLYINORGANICMEMBRANESDUETOTHEIRHIGHERTHERMALSTABILITY,GOODCHEMICALRESISTANCETOSOLVENTS,HIGHMECHANICALSTRENGTHANDLONGLIFETIMESURFACEMODIFICATIONSCANBEUTILIZEDININORGANICMEMBRANESTOFURTHERENHANCETHESELECTIVITY,PERMEABILITYORCATALYTICACTIVITIESOFTHEMEMBRANETHISPAPERISTOPROVIDEACOMPREHENSIVEREVIEWONGASSEPARATION,COMPARINGMEMBRANETECHNOLOGYWITHOTHERCONVENTIONALMETHODSOFRECOVERINGCO2FROMNATURALGAS,CHALLENGESOFCURRENTCOMMERCIALPOLYMERICMEMBRANESANDINORGANICMEMBRANESFORCO2REMOVALANDMEMBRANESURFACEMODIFICATIONFORIMPROVEDSELECTIVITYKEYWORDSMEMBRANETECHNOLOGYINORGANICMEMBRANECO2SEPARATIONNATURALGASSURFACEMODIFICATIONZEEYINGYEOPHDSTUDENTATCHEMICALENGINEERINGDISCIPLINE,SCHOOLOFENGINEERING,MONASHUNIVERSITYSUNWAYCAMPUSSHEISWORKINGONINORGANICANDFUNCTIONALMEMBRANESFORCARBONDIOXIDESEPARATIONTHIAMLENGCHEWASSISTANTPROFESSOROFTHEFACULTYOFENGINEERINGANDGREENTECHNOLOGY,UNIVERSITITUNKUABDULRAHMAN,MALAYSIAHERECEIVEDHISPHDDEGREEINCHEMICALENGINEERINGIN2012HISRESEARCHINTERESTSFOCUSONTHEAREASOFTHESEPARATIONOFCARBONDIOXIDEUSINGINORGANICANDFUNCTIONALMEMBRANESANDDEVELOPMENTOFZEOLITEMATERIALSCORRESPONDINGAUTHORTEL60355146234FAX60355146207EMAILCHAISIANGPIAOMONASHEDUSPCHAITHISWORKWASSUPPORTEDBYTHEMINISTRYOFHIGHEREDUCATIONMALAYSIATHROUGHLONGTERMRESEARCHGRANTSCHEMEA/CNUMBER211022611300COPYRIGHT2012,DALIANINSTITUTEOFCHEMICALPHYSICS,CHINESEACADEMYOFSCIENCESALLRIGHTSRESERVEDDOI101016/S1003995311603666JOURNALOFNATURALGASCHEMISTRYVOL21NO32012283PENGWEIZHUASSOCIATEPROFESSOROFTHESCHOOLOFENGINEERING,MONASHUNIVERSITYSUNWAYCAMPUSHERECEIVEDHISPHDDEGREEINCHEMISTRYIN1994HISMAINRESEARCHINTERESTSAREINFUNCTIONALCOLLOIDS,SURFACTANTSANDEMULSIONS,STIMULIRESPONSIVE/FUNCTIONALMATERIALS,INTERFACIALCONFORMATIONSANDRELEVANTPHENOMENA,NONEQUILIBRIUMANDRELAXATIONSATSURFACES/INTERFACESABDULRAHMANMOHAMEDPROFESSOROFTHESCHOOLOFCHEMICALENGINEERINGANDDIRECTOROFTHECENTREFORENGINEERINGEXCELLENCE,UNIVERSITISAINSMALAYSIAHERECEIVEDHISPHDDEGREEINCHEMICALENGINEERINGIN1993HISRESEARCHINTERESTSFOCUSONREACTIONENGINEERINGANDCATALYSIS,AIRPOLLUTIONANDWASTEWATERCONTROLENGINEERING,FUELTECHNOLOGY,NANOSCIENCEANDNANOTECHNOLOGYSIANGPIAOCHAISENIORLECTUREROFTHESCHOOLOFENGINEERING,MONASHUNIVERSITYSUNWAYCAMPUSHERECEIVEDHISPHDDEGREEINCHEMICALENGINEERINGIN2008HISMAINRESEARCHINTERESTSAREINTHEAREASOFCATALYSISANDREACTIONENGINEERING,MEMBRANETECHNOLOGY,NATURALGASPROCESSINGANDNANOMATERIALSDEVELOPMENT1INTRODUCTION11CO2INNATURALGASTHEREHASBEENINCREASINGDEMANDFORTHEUSEOFNATURALGASASAMOREEFFICIENTANDCLEANERFUELNATURALGASISALSOCONSIDEREDASTHEPRINCIPALFEEDSTOCKFORTHECHEMICALINDUSTRYTHEGLOBALCONSUMPTIONOFNATURALGASISPROJECTEDTOINCREASEFROM95TRILLIONCUBICFEETIN2003TO182TRILLIONCUBICFEETIN20301THECONSUMPTIONOFNATURALGASINTHEUNITEDSTATESISEQUIVALENTTO22TRILLIONCUBICFEETPERYEAR2WHILETHEESTIMATEFORTHENATURALGASCONSUMPTIONINEUROPEANUNIONIS522BILLIONCUBICMETERS3NATURALGASRESERVESINMALAYSIASTOODAT88TRILLIONCUBICFEETASAT1STJANUARY2008,WHICHISTHREETIMESTHATOFCRUDEOILOF546BILLIONBARRELOFTHIS,38ISFOUNDOFFTHEEASTCOASTOFPENINSULARMALAYSIA,48ATOFFSHORESARAWAK,AND14REMAININGATOFFSHORESABAHMALAYSIASGASRESERVESAREEXPECTEDTOLASTFORANOTHER36YEARSWITHCURRENTRATEOFPRODUCTION4CURRENTLY,THEWORLDWIDEMARKETFORNEWNATURALGASSEPARATIONEQUIPMENTCONSUMES5BILLIONPERYEAR,HOWEVER,MEMBRANEPROCESSESONLYHAVELESSTHAN10TOTAL100WATER10000PPMTOTAL100C3CONTENT9501050BTU/SCFDEWPOINT4284ZEEYINGYEOETAL/JOURNALOFNATURALGASCHEMISTRYVOL21NO32012FIGURE1RESERVESTOPRODUCTIONR/PRATIOSOFNATURALGAS5IN2010BYREGIONACIDGASESLIKEH2SANDCO2INNATURALGASWELLSTREAMSHAVENOHEATINGVALUE,ANDTHEYFORMACIDSORACIDICSOLUTIONSINTHEPRESENCEOFWATERWHICHARECORROSIVETHEREMOVALOFCO2ISVERYIMPORTANTASITISONEOFTHEMOSTCOMMONCONTAMINANTSACIDGASESCONTENTCOULDREACHANDEVENOVERPASS50VOLUMEINSOMEUNCONVENTIONALNATURALGASSTREAMS6CO2INTHENATURALGASUSUALLYVARIESFROM450DEPENDINGUPONTHEGASSOURCE8SEPARATIONOFCO2ISIMPORTANTINNATURALGASPROCESSINGBECAUSEITCAUSESSTEELPIPECORROSIONANDCANBLOCKCRYOGENICEQUIPMENTSBYSOLIDIFYINGATLOWTEMPERATURESGENERALLY,PIPELINESPECIFICATIONSFORNATURALGASREQUIREACO2CONCENTRATIONBELOW238BESIDES,THEREMOVALOFCO2WILLALSOINCREASETHECALORIFICVALUEANDTRANSPORTABILITYOFNATURALGAS92CONVENTIONALSYSTEMSOFRECOVERINGCO2FROMNATURALGASTHREEGENERALCATEGORIESOFPROCESSESAVAILABLEFORREMOVALOFCO2FROMREFINERYGASSTREAMSINCLUDECHEMICALSOLVENTS,PHYSICALSOLVENTSANDSOLIDADSORBENTS10CONVENTIONALSYSTEMSOFREMOVINGCO2FROMNATURALGASAREMOSTLYBASEDONCHEMICALORPHYSICALABSORPTION1121CHEMICALABSORPTIONCHEMICALABSORPTIONFORCO2CAPTUREISBASEDONEXOTHERMICREACTIONOFASORBENTWITHCO2PRESENTINTHEGASSTREAMATLOWTEMPERATURETHEREACTIONISTHENREVERSEDINSOCALLEDREGENERATIONATHIGHERTEMPERATUREFORCHEMICALABSORPTION,THELOADINGCAPACITYISLIMITEDBYTHEQUANTITYOFTHEACTIVECOMPONENTOFTHESOLUTIONWHENTHESATURATIONLEVELISREACHED,ONLYAMINORLOADINGCANBEACHIEVEDBYPHYSICALABSORPTIONINTHESOLUTION12CHEMICALSOLVENTSBINDSTRONGLYTOTHECO2,WHICHISEFFECTIVEEVENATLOWPARTIALPRESSURESHOWEVER,THEREGENERATIONOFTHERICHSOLVENTREQUIRESMOREENERGY13INTHECASEOFLOWCO2CONTENTINTHEFEEDANDHIGHPURITYCO2OFTHEPRODUCT,CHEMICALSOLVENTSAREMOSTLYUSED11TWOGROUPSOFCHEMICALSTHATHAVEBEENAPPLIEDTOCO2REMOVALAREAMINESANDPOTASSIUMCARBONATE12211ALKANOLAMINESWEETENINGALKANOLAMINEENCOMPASSESTHEFAMILYOFORGANICCOMPOUNDSOFMONOETHANOLAMINEMEA,DIETHANOLAMINEDEA,TRIETHANOLAMINETEA14,DIISOPROPANOLAMINEDIPA,METHYLDIETHANOLAMINEMDEA15,ANDDIGLYCOLAMINEDGA15AMONGTHESEAMINES,MDEAISMOSTWIDELYUSEDTODAYBECAUSEITISMORESELECTIVETHANOTHERAMINESDUETOCO2BEINGABSORBEDMORESLOWLYTHANHYDROGENSULFIDEH2S,WHICHISCOPRESENTINNATURALGAS12THESEAMINESOLUTIONSCANBEUSEDTOREMOVECO2ANDH2SFROMNATURALGASBYRUNNINGTHESOURGASTHROUGHTHETOWERAMINESOLUTIONSWILLABSORBCO2/H2SASTHESOURGAS,WHICHPASSESTHROUGHANDRESULTSINTHEEFFLUENTGASFREEOFCO2/H2S,ANDTHEREFORELOSESITSSOURGASSTATUS15CONVENTIONALLY,THEFEEDGASENTERSTHEABSORBERFROMTHEBOTTOMWHILETHESOLVENTENTERSTHETOPOFTHEABSORBERTHEN,THEABSORBEDCO2ISSTRIPPEDFROMTHESOLVENTBYCOUNTERFLOWINGSTEAMAT100200CWHENPASSINGTHROUGHAREGENERATORUNITAHIGHCONCENTRATEDCO2STREAMISFORMEDBYCONDENSINGTHEWATERVAPORANDTHELEANSOLVENTISCOOLEDTO4560CANDRECYCLEDBACKTOTHEABSORPTIONCOLUMN16TYPICALREACTIONSOFCO2WITHMEAFORABSORBINGANDREGENERATINGAREASFOLLOWSABSORBINGREACTIONSMEAH2OCO2MEACARBONATEHEAT1REGENERATINGREACTIONSMEACARBONATEHEATMEAH2OCO22THEAMINEBASEDABSORPTION/STRIPPINGPROCESSHASBEENUSEDFORTHESEPARATIONOFCO2FROMNATURALGASANDFLUESTREAMSFOROVER60YEARSANDISREGARDEDASTHEMOSTMATURETECHNOLOGY16ALTHOUGHITHASWIDECOMMERCIALUSE,THISTECHNIQUEHASSEVERALDRAWBACKSSUCHASLOWCO2LOADINGCAPACITY,HIGHEQUIPMENTCORROSION,HIGHENERGYCONSUMPTIONDURINGSOLVENTREGENERATION,AMINEDEGRADATIONBYSO2,NO2,HCL,HF,ANDO2INFLUEGAS16,HIGHSOLUTIONCIRCULATIONRATEANDSOLUTIONDEGRADATION17212CARBONATEANDWATERWASHINGPROCESSESCARBONATEWASHINGISACHEMICALCONVERSIONPROCESSWHERETHECONTAMINANTSINNATURALGASARECONVERTEDTOCOMPOUNDSTHATCANBEREMOVEDFROMTHESTREAMWITHGREATEREASETHANORIGINALCONSTITUENTSTHEMOSTCOMMONPROCESSISTHEABSORPTIONOFCO2BYPOTASSIUMCARBONATECARBONATEWASHINGUSESTHEPRINCIPLETHATTHERATEOFABSORPTIONOFCO2BYPOTASSIUMCARBONATEINCREASESWITHTEMPERATUREANDTHEPROCESSWORKSBESTNEARTHETEMPERATUREOFREVERSIBILITYOFTHEREACTIONS15INTHECARBONATEPROCESS,THESOURGASISHEATEDBYAHEATEXCHANGERANDTHENENTERSTHEBOTTOMOFTHEABSORBERTOBEABSORBEDCOUNTERCURRENTLYBYTHEDESCENDINGLEANHOTCARBONATESTREAMTHEN,THESWEETGASEXITSTHETOPOFTHEABSORBERWHILETHEACIDRICHCARBONATESTREAMISSTRIPPEDTOREMOVETHECO2ABSORBEDANDRECYCLEDBACKTOTHEABSORBERJOURNALOFNATURALGASCHEMISTRYVOL21NO32012285THEOPERATIONTEMPERATUREOFTHEABSORBERISAROUND110CTHECHEMISTRYCANBEREPRESENTEDBYTHEEQUATIONBELOWK2CO3CO2H2O2KHCO3322PHYSICALABSORPTIONPHYSICALABSORPTIONDEPENDSONITSLOADINGCAPACITYWHERETHELOADINGCAPACITYISDIRECTLYPROPORTIONALTOTHEPARTIALPRESSUREOFTHECOMPONENTTOBEREMOVEDTHESOLUBILITYOFCO2WITHINTHESOLVENTSDEPENDSBOTHONTHEPARTIALPRESSUREANDTHETEMPERATUREOFTHEFEEDGASWHEREITFAVORSTHEHIGHERCO2PARTIALPRESSUREANDLOWERTEMPERATURE16PHYSICALSOLVENTSUSUALLYHAVEAWEAKERAFFINITYTOWARDSACIDGASWHICHMEANSTHELEANSOLVENTDOESNOTRAPIDLYABSORBTHEACIDGASESBUTREGENERATIONOFTHERICHSOLVENTREQUIRESLESSENERGY13PHYSICALSOLVENTSAREUSUALLYFAVOREDINTHECASEOFHIGHCO2CONTENTINTHEFEEDGASANDLOWPURITYREQUIREMENTSINTHEPRODUCT11221RECTISOLPROCESSTHERECTISOLPROCESSISCONSIDEREDASONEOFTHEOLDESTCOMMERCIALACIDGASREMOVALSWHICHARESTILLINUSE13ITWASLICENSEDBYTHELINDECORPORATIONGMBH18COLDMETHANOLISUSEDASSOLVENTINRECTISOLPROCESSANDISORIGINALLYDEVELOPEDTOPROVIDETREATMENTFORGASFROMTHELURGIMOVINGBEDGASIFIER,WHICHCONTAINSHYDROCARBONS,AMMONIA,HYDROGENCYANIDEANDOTHERIMPURITIESINADDITIONTOCO2ANDH2S12THEOPERATINGTEMPERATURERANGEISBETWEEN29CTO59C19THISPROCESSTECHNIQUEADAPTSAPRELIMINARYCOLDMETHANOLWASHOFTHEFEEDGASTOREMOVEWATERINTHEGASSTREAMBEFOREITENTERSTHEABSORBERTHISISFOLLOWEDBYCHILLINGTHEGASMIXTURESOUTOFGASPHASEBYTHECOLDMETHANOLSOLVENTINSIDETHEABSORBERTHEN,BYEMPLOYINGASTEAMHEATEDREBOILERINTHESTRIPPER,THEREGENERATIONOFLEANMETHANOLSOLVENTTAKESPLACE18MANYPOSSIBLEPROCESSCONFIGURATIONSFORRECTISOLPROCESSEXISTSDEPENDINGONPROCESSREQUIREMENT,SPECIFICATIONSANDSCALABILITYTHISPROCESSISEXTENSIVELYUSEDINNATURALGASINDUSTRYTOREMOVECO2THEDRAWBACKSOFRECTISOLINCLUDETHECOMPLEXITYOFTHEPROCESSES,EXTENSIVEUSEOFREFRIGERATIONSWHICHRESULTSINRECTISOLASANEXPENSIVEPROCESS13,1516,20,LOWTEMPERATUREWHICHLEADSTOTHERMALLOSS17,ANDCHILLEDMETHANOLSOLVENTWHICHCOULDABSORBMETALLICTRACECOMPONENTSSUCHASMERCURYTOFORMAMALGAMSINTHEPROCESS16222SELEXOLSINCE1969,SELEXOLHASBEENUSEDTOSWEETENNATURALGAS,BOTHFORBULKCO2REMOVALANDH2SREMOVAL16CURRENTLY,THESELEXOLPROCESSISOWNEDBYUNIVERSALOILPRODUCTSBUTTHISPROCESSWASORIGINALLYDEVELOPEDBYALLIEDCHEMICALCORPORATION21SELEXOLPROCESSUSESDIMETHYLETHERSOFPOLYETHYLENEGLYCOLDMPEG,AGLYCOLBASEDSOLVENTWHICHISEFFECTIVEINCAPTURINGBOTHCO2ANDH2SATHIGHERCONCENTRATIONITHASAVERYLOWVAPORPRESSUREANDHIGHCAPACITYFORVARIOUSIMPURITIESSUCHASH2S,CO2,CARBONYLSULFIDEANDMERCAPTANS21THEIROPERATINGTEMPERATURERANGEIS040C12THISOPERATINGTEMPERATURERANGESOFFERSUBSTANTIALLYREDUCEDCOSTSBYELIMINATINGORREDUCINGTHEREFRIGERATIONDUTY19THEABSORPTIONTEMPERATURETAKESPLACEAROUND05CWHILEDESORPTIONOFTHERICHSOLVENTCANBEDONEBYLETTINGDOWNTHEPRESSURE,ORBYSTRIPPINGWITHINERTGAS,AIRORSTEAM16ALTHOUGHSELEXOLWASUSEDSINCE1969TOSWEETENNATURALGAS,ITHASSEVERALDRAWBACKSSUCHASEXPENSIVESOLVENTS,HIGHCAPITALCOSTSFOREQUIPMENT22,HIGHCIRCULATIONRATE,HIGHSULFUROUTLETCONTENT17ANDUNDESIREDABSORPTIONOFHIGHERHYDROCARBONSWITHCO2WHICHRESULTSINHYDROCARBONLOSSES16,2323PHYSICALCHEMICALABSORPTIONPHYSICALCHEMICALABSORPTIONGENERALLYUSESACOMBINATIONOFAMINEANDOTHERORGANICPHYSICALSOLVENTS,WHICHCANUSUALLYACCEPTAHIGHERLOADINGTHANANAQUEOUSAMINESOLUTION,THEREBYREDUCINGSOLVENTRATES12231SULFINOLPROCESSTHESULFINOLPROCESSISDEVELOPEDIN1960S15ANDLICENSEDBYTHESHELLINTERNATIONALPETROLEUMMAATSCHAPPIJSIPMINNETHERLANDSANDSHELLOILCOMPANYINTHEUS21THESULFINOLPROCESSUSESAMIXTUREOFSOLVENTSCOMPOSEDOFSULFOLANE,DIISOPROPANOLAMINEDIPAANDWATER,ALLOWINGITTOBEHAVEASBOTHACHEMICALANDPHYSICALSOLVENTPROCESSCOMMONSULFINOLMIXTURESARECOMPOSEDOF40AMINEDIPA,40SULFOLANEORGANICSOLVENT,AND20WATER15THESULFOLANEACTSASTHEPHYSICALSOLVENTWHILEDIPAACTSASTHECHEMICALSOLVENT7,14INTHISMIXEDSOLVENTPROCESS,BULKOFTHECO2WILLBEREMOVEDBYTHEPHYSICALSOLVENTWHILETHEPROCESSGASISPURIFIEDBYTHECHEMICALSOLVENTTOSTRINGENTLEVELS,ALLINASINGLESTEP21THESULFINOLDPROCESSUSESDIISOPROPANOLAMINEDIPAWHILESULFINOLMONEUSESMETHYLDIETHANOLAMINEMDEA15WHENCOMPLETEREMOVALOFBOTHH2SANDCO2ANDDEEPREMOVALOFCARBONSULFIDEAREDESIRED,SULFINOLDISUSEDWHENSELECTIVEREMOVALOFHYDROGENSULFIDEOVERCO2ANDPARTIALREMOVALOFCARBONYLSULFIDEISREQUIRED,SULFONILMISUSED21THEOPERATIONPROCESSOFSULFINOLISVERYSIMILARTOTHEAMINEMETHODEVENITSEQUIPMENTCOMPONENTSARESIMILARTOAMINEUNITSHOWEVER,THEMAINDIFFERENCEISTHATTHECONVENTIONALAMINEPROCESSEMPLOYSDILUTEDCONCENTRATIONOFAMINEINWATERTOREMOVEACIDGASESBYCHEMICALREACTION,WHILETHESULFINOLSYSTEMUSESMIXTUREOFHIGHLYCONCENTRATEDAMINEANDPHYSICALSOLVENTTOREMOVEACIDGASESBYPHYSICALANDCHEMICALREACTIONSTHEADVANTAGESOFSULFINOLPROCESSINCLUDELOWSOLVENTCIRCULATIONRATESWITHSMALLEREQUIPMENTANDLOWERCOSTANDTHEDISADVANTAGEISTHEABSORPTIONOFHEAVYHYDROCARBONSANDAROMATICS7,14286ZEEYINGYEOETAL/JOURNALOFNATURALGASCHEMISTRYVOL21NO3201224ADSORPTIONPROCESSADSORPTIONISTHEENRICHMENTOFGASEOUSORDISSOLVEDSOLVENTSONTHEBOUNDARYSURFACEOFASOLIDTHESESURFACESHAVESOCALLEDACTIVESITESWHICHCANBINDFOREIGNMOLECULESDESORPTIONISTHEREVERSALOFADSORPTIONPROCESSWHERETHEADSORBEDMOLECULESISLIBERATED24THECAPABILITYOFADSORBENTONCARRYINGTHEQUANTITYOFCO2COMPONENTDEPENDSNOTONLYONTHECHARACTERISTICSOFCOMPONENTANDSORBENT,BUTALSOONTHETEMPERATUREANDPRESSUREUNDERWHICHITTAKESPLACEBOTHTEMPERATUREANDPRESSUREEFFECTSAREUSEDFORTHECLASSICADSORPTIONDESORPTIONCYCLE,WHEREHIGHPRESSUREANDLOWTEMPERATUREAREUSEDFORADSORPTIONWHILELOWPRESSUREANDHIGHTEMPERATUREAREUSEDFORDESORPTION12PHYSICALADSORBENTSSUCHASCARBONBASEDMATERIAL,ZEOLITESANDMETALORGANICFRAMEWORKMOFMOSTLYADSORBWATERVAPORPREFERENTIALLYOVERCO2ANDTHEIRCO2ADSORPTIONCAPACITYATLOWPRESSUREISNOTSUFFICIENTLYHIGHHOWEVER,EFFORTSLIKESURFACEMODIFICATIONCANBEDONETOENHANCETHEINTERACTIONSWITHCO2,THUSINCREASINGADSORPTIONCAPACITYATLOWPRESSUREANOTHERMETHODISTODESIGNCOMPLETELYNEWMATERIALSSUCHASCOVALENTORGANICFRAMEWORKSANDZMOFSTOINCREASETOLERANCETOMOISTUREINTHEGASFEED,ANDINDIRECTLYIMPROVECO2ADSORPTIVITY25241MOLECULARSIEVEADSORBENTTHEMOSTCOMMONAPPLICATIONOFMOLECULARSIEVESINCONNECTIONWITHGASIFICATIONPLANTSISTHEREMOVALOFWATERANDCO2UPSTREAMOFCRYOGENICUNITSTHESEPROCESSES,CRYOGENICGASSEPARATIONANDAIRSEPARATION,WORKINGATCRYOGENICTEMPERATURES,REQUIREAFEEDGASCOMPLETELYFREEOFWATERANDCO2,WHICHWOULDOTHERWISEFREEZEANDDEPOSITONTHEINLETHEATEXCHANGERSANDEVENTUALLYBLOCKTHEM12MOLECULARSIEVEADSORBENTSHAVEWIDEAPPLICATIONSWHICHINCLUDEGASSEPARATION,DRYINGANDPURIFYINGSEVERALWELLKNOWNMOLECULARSIEVINGADSORBENTSAREZEOLITES,ALUMOPHOSPHATESANDCARBONMOLECULARSIEVESETC,WHILEOTHERNEWTYPESOFMOLECULARSIEVEADSORBENTSAREMETALORGANICFRAMEWORKSOFMILTYPE,MESOPOROUSSILICATES,ANDCARBONSETC26MOLECULARSIEVESARESPECIALLYDESIGNEDTOSEPARATEVARIOUSMOLECULESBASEDONTHEIRMOLECULARWEIGHTORMOLECULARSIZE27THESELECTIVITYANDRETENTIONOFADSORBEDCOMPOUNDSAREDIRECTLYPROPORTIONALTOTHEIRPOLARITYANDEFFECTIVEMOLECULARSIZEWHICHISRATIONALEFOR“MOLECULARSIEVE”28MANYRESEARCHACTIVITIESAREDONEWITHTHEPURPOSEOFIMPROVINGTHECO2ADSORPTIONBYCHEMICALMODIFICATIONOFMOLECULARSIEVESURFACE16,27THEMAININTERESTISFOCUSEDONTHEAMINESORBENTSWHICHINCORPORATEBASICORGANICGROUPSONTOSURFACEOFHIGHSPECIFICAREASOLIDSSUCHASMICROORMESOPOROUSSILICA,ALUMINAORPOLYMERBASEDMATERIALSAMINESORBENTSHAVEBEENUSEDSINCEEARLY1990SFORCO2REMOVALINSPACESHUTTLELIFESUPPORTSYSTEMS,WITHREGENERATIONBYVACUUMSWINGADSORPTION29THEREACTIONBETWEENTHEACIDICCO2MOLECULESANDBASICSURFACEWILLRESULTINTHEFORMATIONOFSURFACEAMMONIUMCARBAMATEUNDERANHYDROUSCONDITIONSANDTHEFORMATIONOFAMMONIUMBICARBONATEANDCARBONATESPECIESINTHEPRESENCEOFWATERSILICA,SBA1,SBA15,MCM41,ANDMCM48AREATTRACTIVEMESOPOROUSSUBSTRATESBECAUSETHEYPOSSESSPORESLARGEENOUGHTOBEACCESSEDBYMOLECULESWITHAMINEGROUPSTHEPOROSITYANDSURFACEFUNCTIONALGROUPSBOTHFACILITATETHECAPTUREOFCO216,27242CO2ADSORPTIONBYACTIVATEDCARBONACTIVATEDCARBONSAREAPPLIEDINAWIDERANGEOFINDUSTRIALANDTECHNOLOGICALPROCESSESWITHITSWELLDEVELOPEDMICROANDMESOPOROSITIES16MOLECULESFROMTHELIQUIDORGASPHASESCANBEBOUNDONTOTHESURFACEOFACTIVATEDCARBONBYPREDOMINANTLYPHYSICALFORCESOFVANDERWAALSTYPEMOREOVER,CHEMISORPTIONSCOULDALSOBECAUSEDBYSTRONGERVALENCEFORCESONTHEACTIVESITESOFTHECARBONSURFACEALTHOUGHPOROSITYANDSURFACEAREAAREPARAMETERSDEFININGTHEQUALITYOFACTIVATEDCARBON,SURFACECHEMISTRYOFTHECARBONSALSOPLAYANIMPORTANTROLEINTHEIRADSORPTIVEPROPERTIES30CARBONADSORBERSUSUALLYOPERATEDATTEMPERATURELOWERTHANABOUT52CBECAUSETHECAPTUREANDRETENTIONOFORGANICCONTAMINANTSAREHIGHERATLOWERTEMPERATURES31COMMERCIALACTIVATEDCARBONSHAVESURFACEAREAUSUALLYLIMITEDTOLESSTHAN1200M2/G32THEFUNDAMENTALSOFCO2ADSORPTIONINACTIVATEDCARBONSHAVEGAINEDMOREATTENTION33SELECTIVEADSORPTIONOFCO2ONACTIVATEDCARBONISUSEDCOMMERCIALLYFORSEPARATIONOFBULKCO2FROMGASMIXTUREORTRACECO2FROMACONTAMINATEDGAS34PHYSICALADSORPTIONCAPACITYOFCO2ONACTIVATEDCARBONSCANBEENHANCEDBYINTRODUCINGNITROGENFUNCTIONALGROUPSINTOTHEIRSTRUCTURETHISCANBEACHIEVEDBYIMPREGNATINGTHESURFACEWITHAPPROPRIATECHEMICALSLIKEPOLYETHYLENIMINEPEIORINTRODUCINGNITROGENINTOTHECARBONSTRUCTURE16BESIDES,IMPREGNATIONWITHAMINES,HEATTREATMENTINPRESENCEOFAMMONIAGASANDFUNCTIONALIZATIONWITHAMINOGROUPSBYELECTROPHILICAROMATICCANALSOBEDONETOIMPROVECO2ADSORPTIONCAPACITY34DESPITEITSUSEFULNESSININDUSTRIALAPPLICATION,ADSORPTIONBYACTIVATEDCARBONSTILLCOVERSSEVERALDISADVANTAGESWHICHINCLUDETHELIMITATIONTOVOLATILEORGANICS,LOWREMOVALCAPACITY,HIGHEROPERATINGCOSTS,ANDPOTENTIALFORFIREATTHEBED31243COMPARISONOFMEMBRANETECHNOLOGYANDOTHERMETHODSFORCO2REMOVALABSORBERSTRIPPERUNITSHAVEBEENPROVENTOBEAWELLACCEPTEDTECHNOLOGYINTHEGASPROCESSINGINDUSTRYHOWEVER,ABSORBERTOWERISINPARTICULAREXPENSIVE,LARGE,THICKWALLEDANDHEAVYVESSELSBESIDES,LARGEAMOUNTSOFABSORBENTFLUIDMUSTBEUSEDANDHIGHMAINTENANCESARENEEDEDTOKEEPTHEABSORBERSTRIPPERUNITSINGOODCONDITION2ONTHEOTHERHAND,ADSO
- 温馨提示:
1: 本站所有资源如无特殊说明,都需要本地电脑安装OFFICE2007和PDF阅读器。图纸软件为CAD,CAXA,PROE,UG,SolidWorks等.压缩文件请下载最新的WinRAR软件解压。
2: 本站的文档不包含任何第三方提供的附件图纸等,如果需要附件,请联系上传者。文件的所有权益归上传用户所有。
3.本站RAR压缩包中若带图纸,网页内容里面会有图纸预览,若没有图纸预览就没有图纸。
4. 未经权益所有人同意不得将文件中的内容挪作商业或盈利用途。
5. 人人文库网仅提供信息存储空间,仅对用户上传内容的表现方式做保护处理,对用户上传分享的文档内容本身不做任何修改或编辑,并不能对任何下载内容负责。
6. 下载文件中如有侵权或不适当内容,请与我们联系,我们立即纠正。
7. 本站不保证下载资源的准确性、安全性和完整性, 同时也不承担用户因使用这些下载资源对自己和他人造成任何形式的伤害或损失。

人人文库网所有资源均是用户自行上传分享,仅供网友学习交流,未经上传用户书面授权,请勿作他用。
 川公网安备: 51019002004831号
川公网安备: 51019002004831号