新型电动自行车及动力反馈制动系统设计
47页 27000字数+说明书+外文翻译+10张CAD图纸【详情如下】
刹车上触压片.dwg
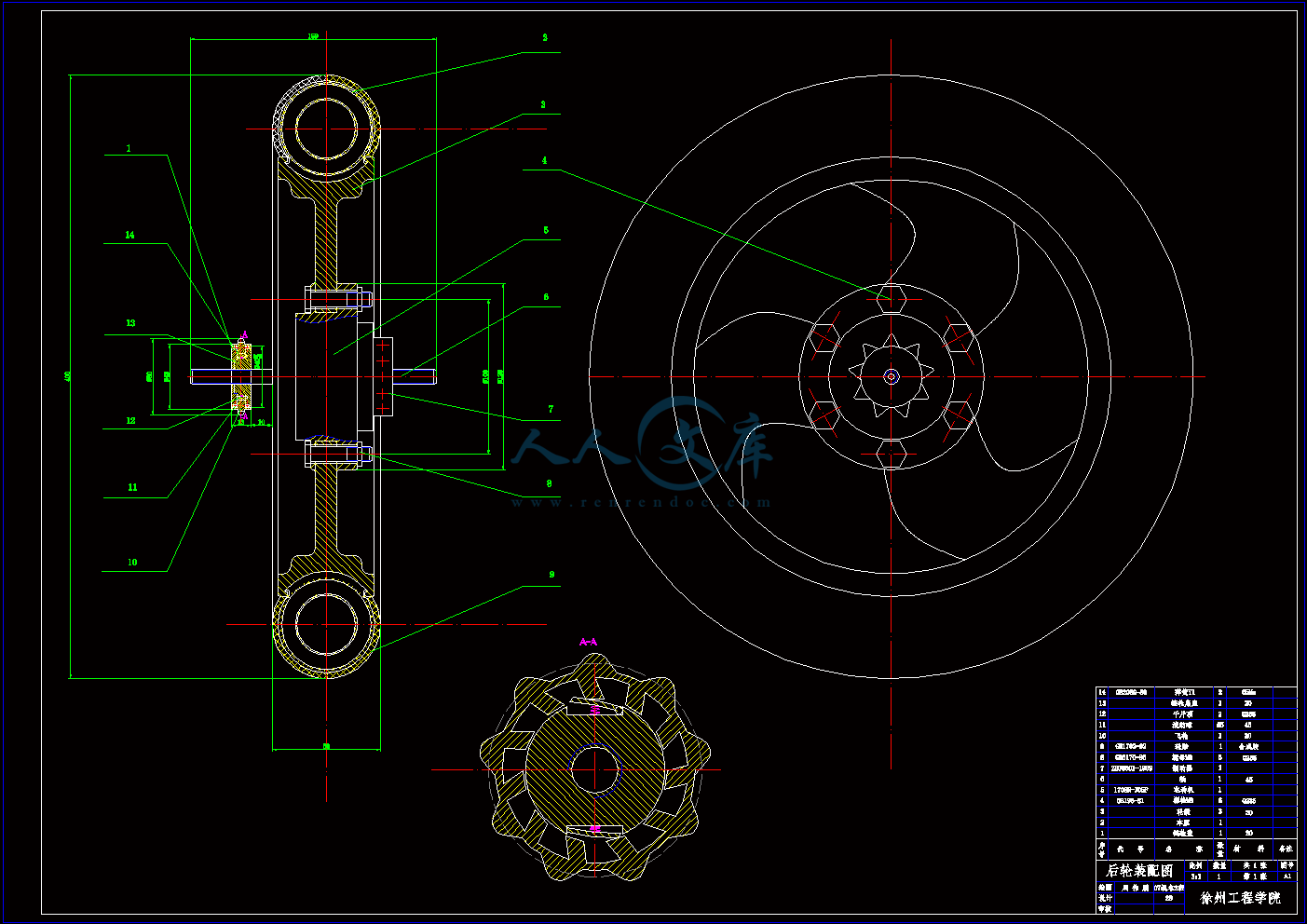
后轮装配图.dwg
外文翻译--阀控式铅酸蓄电池的失效模式在深放电电动自行车的应用 中文版.doc
外文翻译--阀控式铅酸蓄电池的失效模式在深放电电动自行车的应用 英文版.pdf
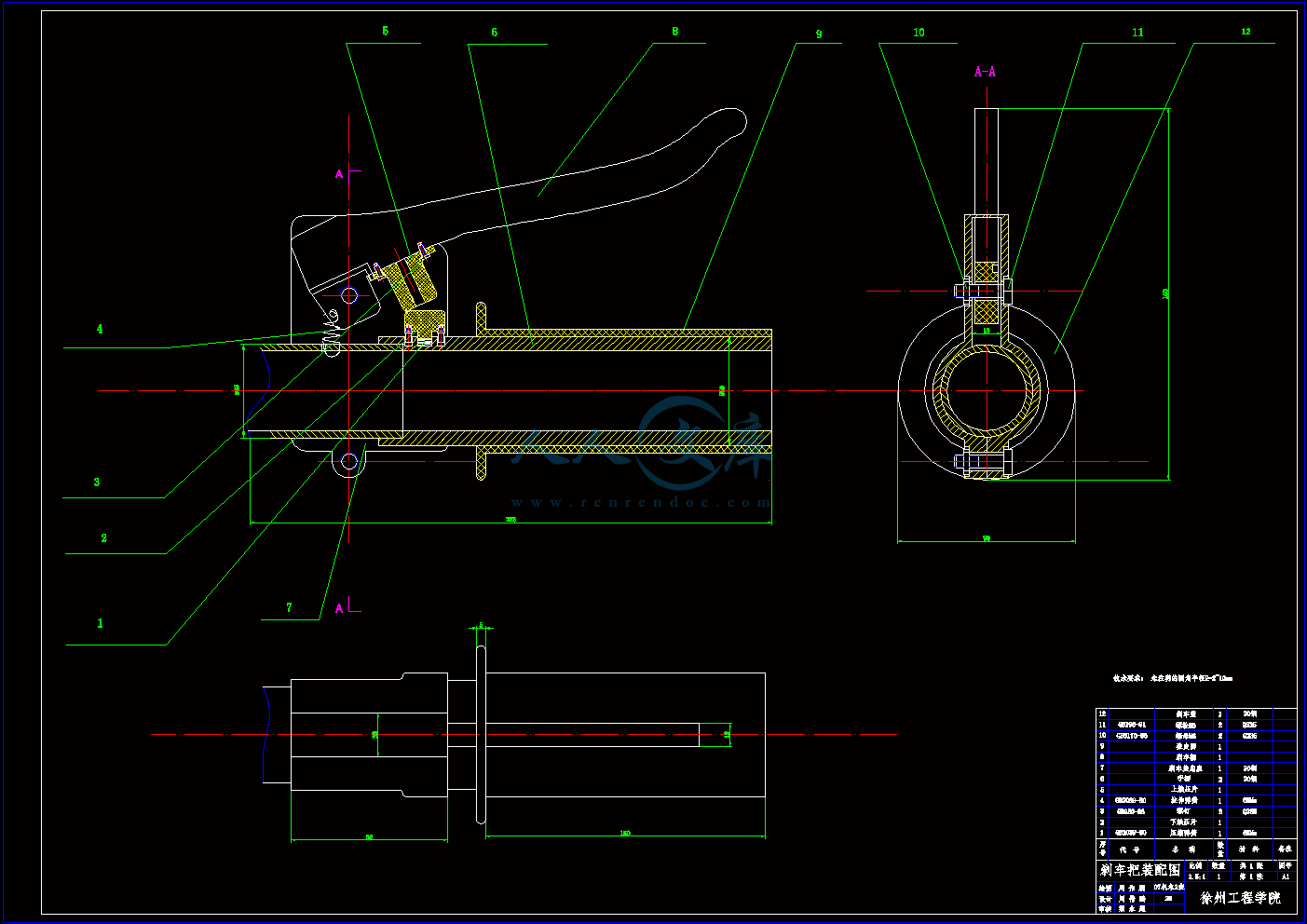
左刹车把装配图.dwg
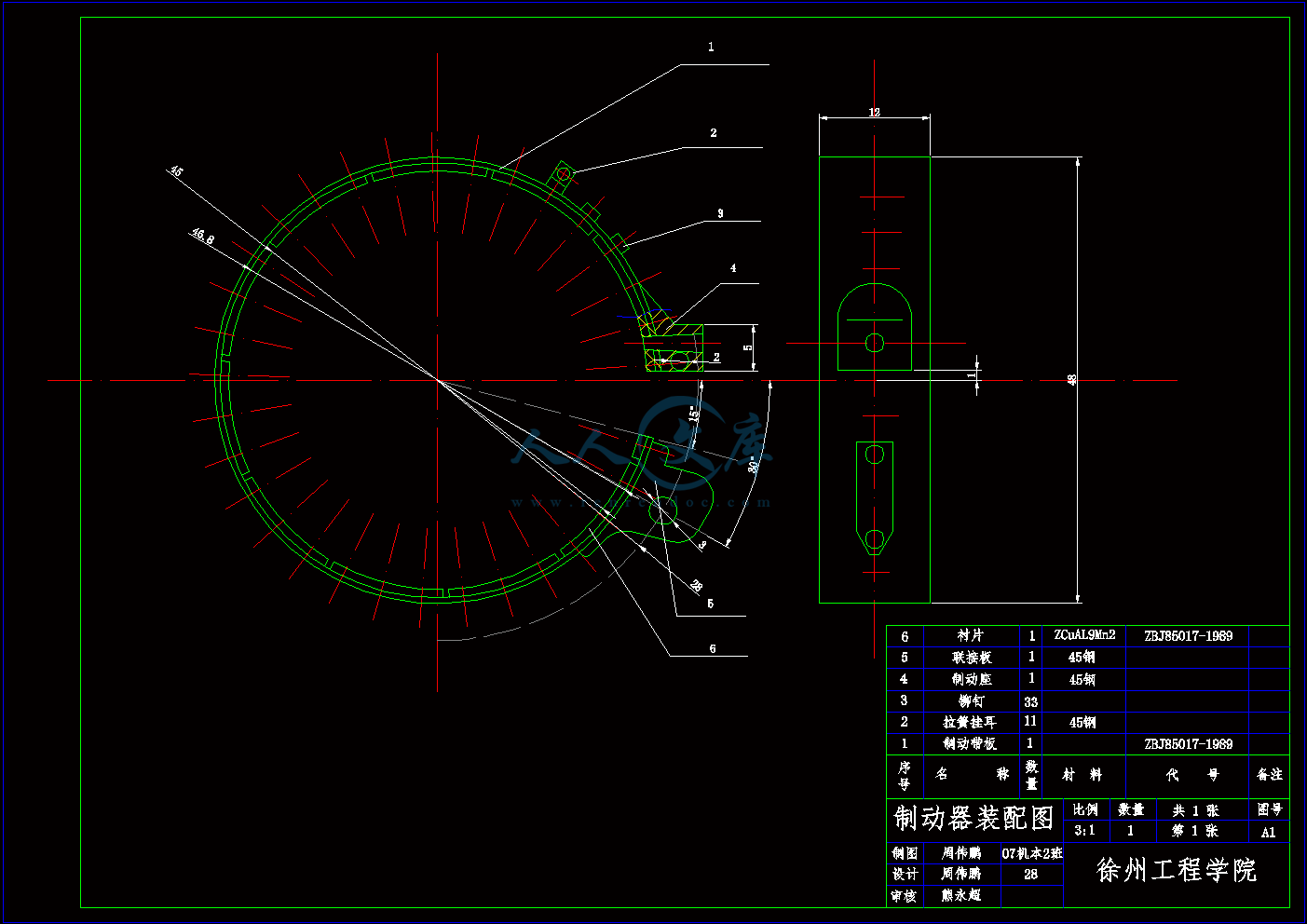
带式制动器.dwg
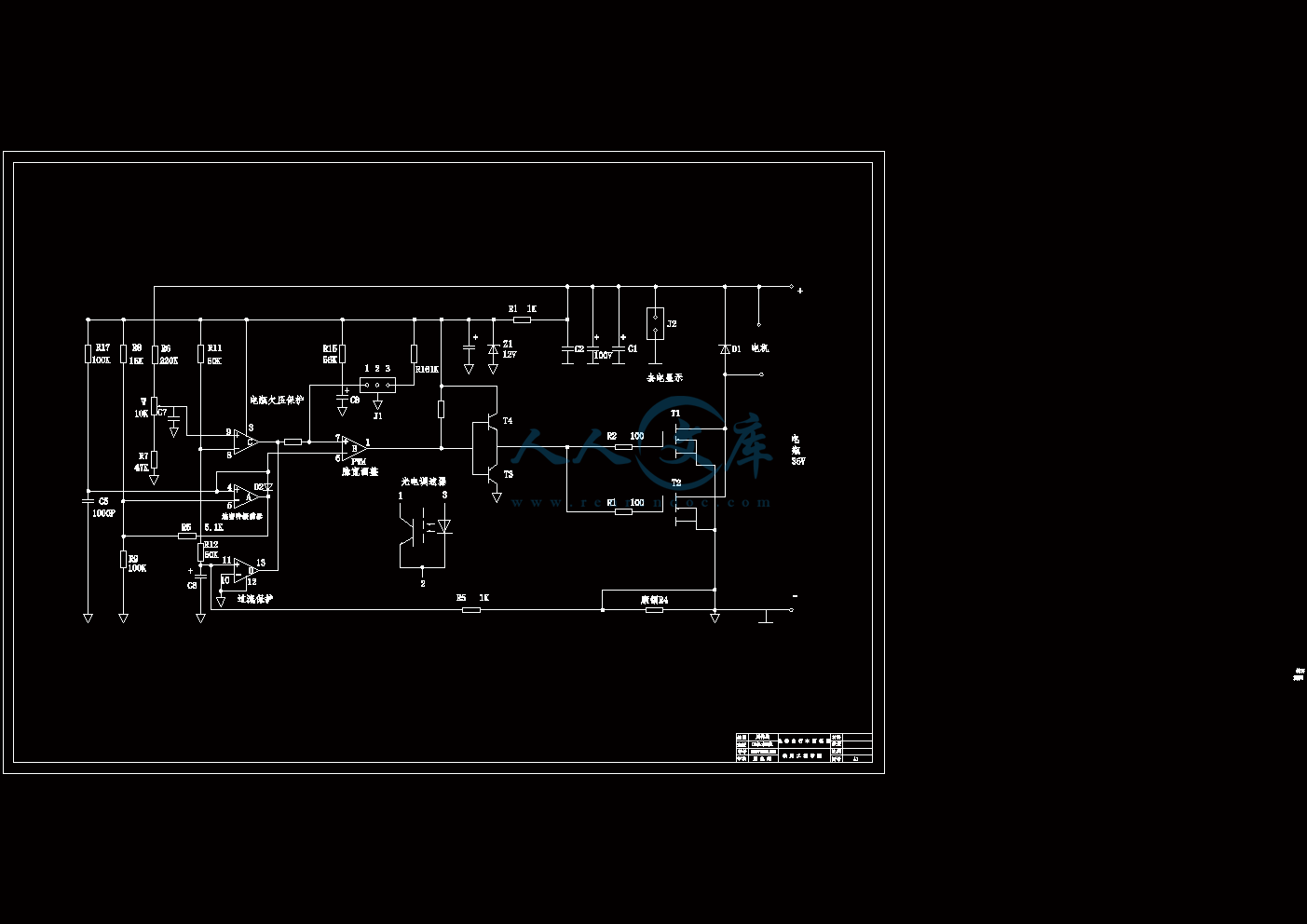
控制线路图.dwg
新型电动自行车及动力反馈制动系统设计说明书.doc
电动自行车原理图.dwg
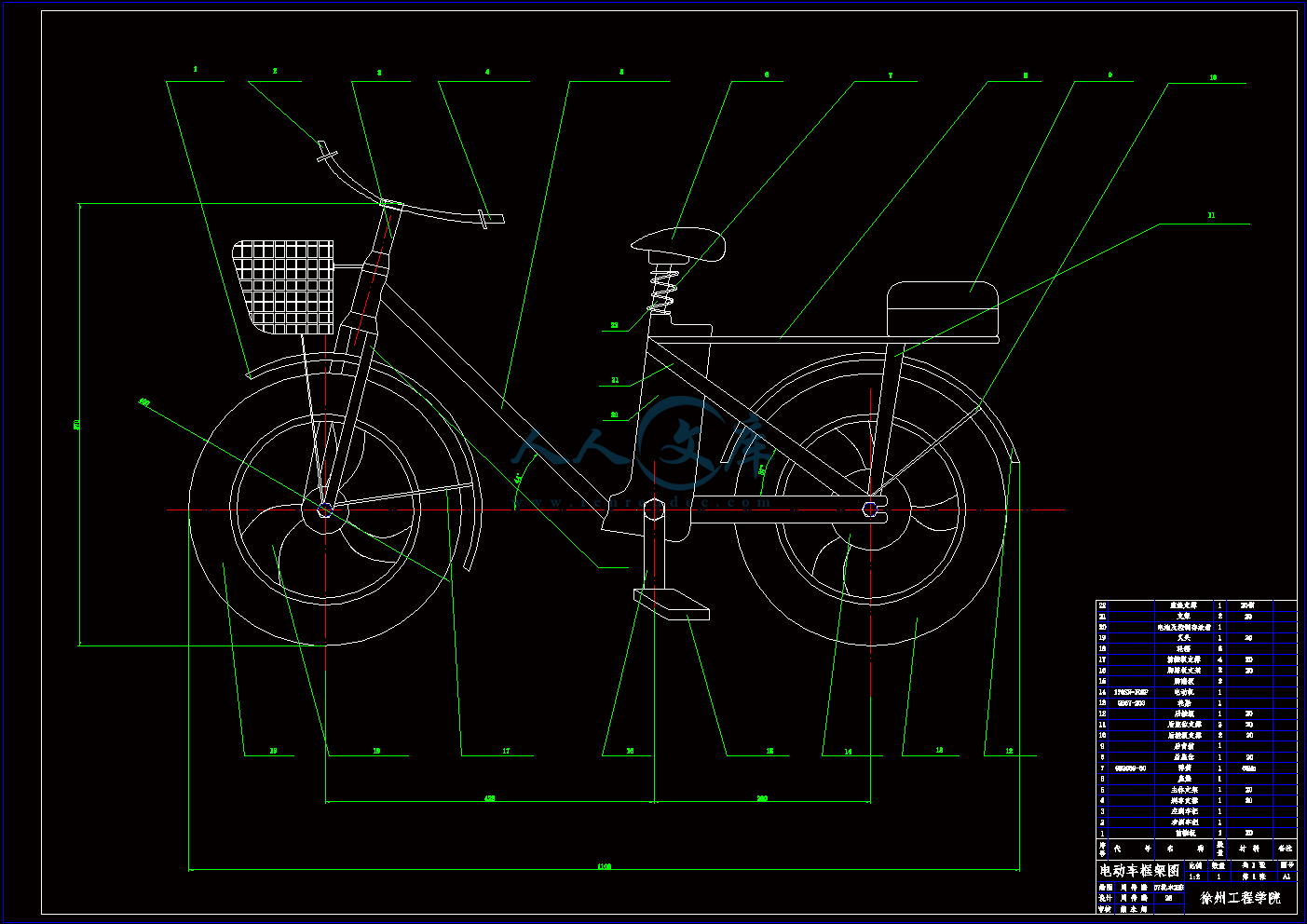
电动自行车框架图.dwg
论文.doc
链轮底座.dwg
链轮盖.dwg
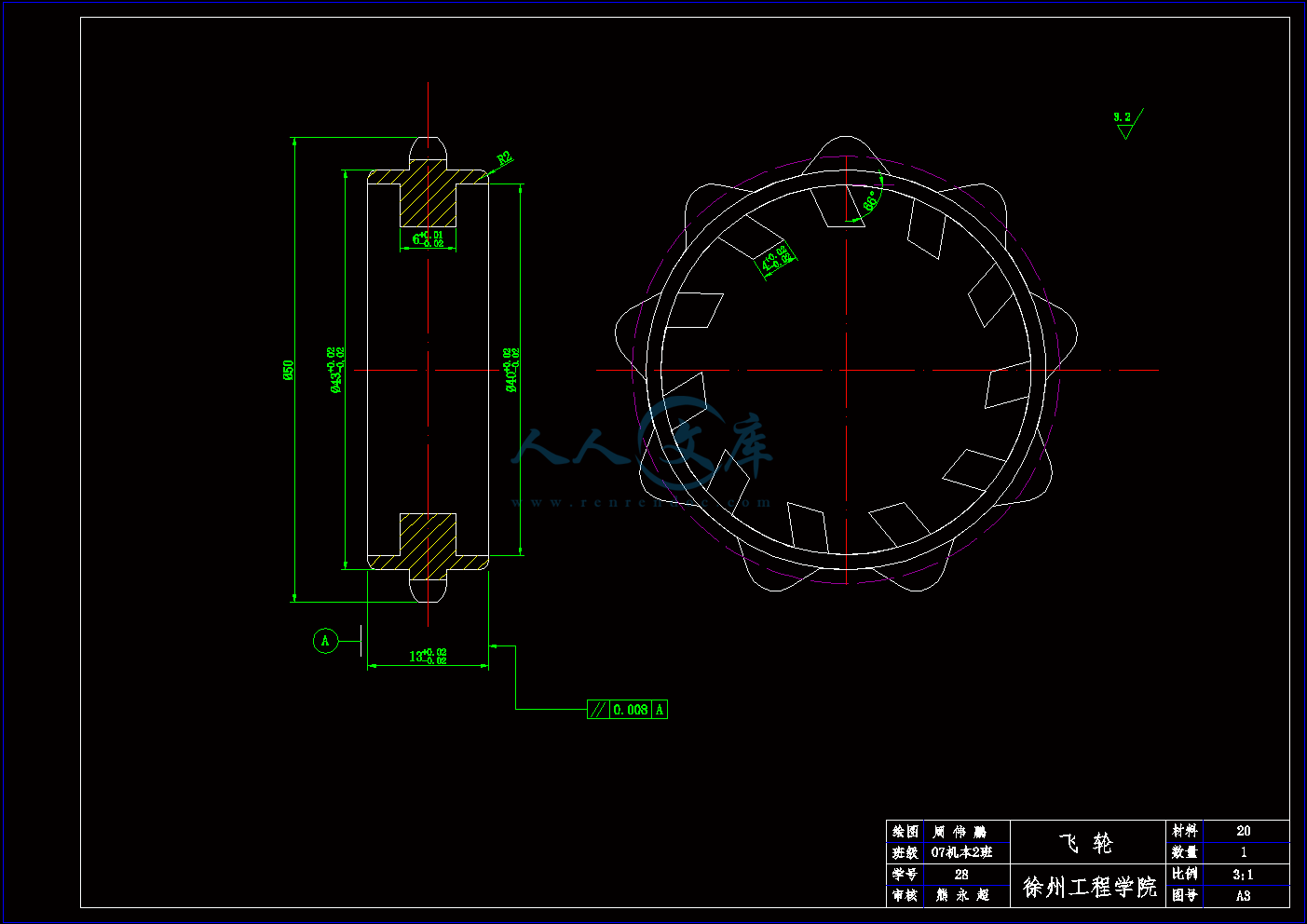
飞轮.dwg





目 录
摘要I
AbstractII
1 绪论1
1.1 课题来源,目的,意义与该课题的市场前景1
1.2 有关电动自行车的概念1
1.2.1 什么是电动自行车1
1.2.2 电动自行车的种类1
1.2.3 电机2
1.2.4 电池2
1.2.5 控制器3
1.3 电动自行车整车参数的设计3
1.4 设计任务3
1.5 研究意义4
2 电动自行车动力性能分析5
2.1 电动车运行方程5
2.2 电动自行车的行驶力学6
2.2.1 车辆模型6
2.2.2 电动车阻力计算7
2.2.3 空气阻力8
2.2.4 电动车惯性力的计算8
2.2.5 电动车的牵引力计算9
2.3 电动自行车的动力性能10
2.3.1 自行车基本参数介绍11
2.4 制动系统11
2.4.1 电动自行车刹车分类11
2.4.2 制动力的分析与求解11
2.4.3 手动制动器的设计12
2.5 蓄电池的种类和结构14
2.6 动力传动系统设计15
2.6.1 驱动方式对两轮电动车性能的影响15
2.6.2 电动自行车驱动系统的构成16
2.6.3 无刷直流电动机驱动系统16
3 电动自行车的运行控制18
3.1 电动机的选择18
3.1.1 直流电动机的特点18
3.1.2 电动机容量选择的原则19
3.1.3 电动机的发热与冷却19
3.1.4 选择步骤:20
3.1.5 无刷直流电动轮毂的选型计算20
3.1.6 电动自行车的续行距离21
3.2 无刷电动机的调速控制系统22
3.2.1 直流电动机的机械特性22
3.2.2 脉宽调制(PWM)23
3.2.3 传感器23
3.3 电动车控制系统设计24
3.3.1 控制系统的组成24
3.3.2 控制系统设计方案24
4 电动自行车的能量回收28
4.1 制动模式与能量的分析28
4.2 能量回馈的控制策略29
4.3 电动自行车能量的消耗评价方法29
4.3.1 能量流分配关系及能量测量30
4.3.2 能耗影响因素分析30
4.4 制动效能及制动能量回收的约束条件30
4.4.1 自行车的制动效能30
4.5 制动能量回收控制算法31
4.5.1 制动能量回收的约束条件31
4.6 永磁无刷直流电机相关性能及其能量回馈制动原理32
4.6.1 永磁无刷直流电机及其基本工作原理32
4.6.2 直流电动机的制动方式33
4.6.3 永磁无刷直流电机能量回馈制动原理35
结论39
致谢40
参考文献41
电动自行车整车参数的设计
在设计前先将文章中计算需要的数据假定如下:
整车质量() 50
重力加速度() 10
滚动阻力系数()
人重() 70
车轮直径() 400
后轴转子直径() 10
1.4 设计任务
对电动自行车的运行状态进行了分析,分别对电动自行车的运行阻力、惯性力、再生制动力以及所需要的牵引力进行计算。
利用现代电子技术及电动机发电反馈制动理论,设计一套能够在电气制动的同时,发电给电瓶充电的发电反馈制动系统。
利用该系统能够实现将自行车及人体的动能通过电动机的发电运行状态转化为电能,并经过相应的处理和控制电路,达到给电瓶充电和自行车减速的双重目的。
完成发电反馈制动系统机械连动机构、发电反馈制动电控系统、发电反馈回路的设计、电动机选型。
1 绪论
1.1 课题来源,目的,意义与该课题的市场前景
当今时代,电动自行车已经成为人们日常生活不可缺少的代步工具,改变了人们的生活方式提高了人们的生活质量。自上世纪以来,汽车工业的发展就开始面临两大难以回避的挑战:能源短缺与环境保护。根据目前世界石油总储量和年产量计算,石油资源最多再能支持三四十年的工业消费,再加上政治、局部战争等不稳定因素对能源安全的影响,汽车的燃料供应存在着潜在的隐患。此外汽车废气造成的大气污染日益严重。针对上述问题,现在电动汽车、电动自行车已经进入中国市场。
该课题的目的是通过对电动自行车制动能量的回收过程进行分析和研究,为提高制动能量回收能力提出解决方案。
该课题的意义:首先本设计的课题使我们对电动自行车有了进一步的了解,对制动能量再生有了新的认识,所谓制动能量再生,是指电动自行车减速制动时将其中一部分机械能转化为其他形式的能量这里是电能,并且将其充进电池。制动能量再生方法原理是先将电动自行车制动或减速时的一部分机械能转化为电能存储在电池中,同时产生一定的负荷阻力使电动自行车速度降低;当电动车再次起动或加速时再生系统又将存储的能量再次转化为电动自行车行驶时所需要的动能。这样就可以提高一次充电的续行距离,避免了不必要的能量损失。
1.2 有关电动自行车的概念
1.2.1 什么是电动自行车
电动自行车是以电池作为辅助能源,具有两个车轮,能实现人力骑行、电动或电助动功能的特种自行车。它虽然具有普通自行车的外表特征,但更主要的是,它是在普通自行车的基础上,安装电动机、控制器、电池、转把、闸把等操纵部件和显示仪表系统的、机电一体化的个人交通工具。
1.2.2 电动自行车的种类
电动自行车以其驱动力性质、整车结构及电动机驱动方式的不同,可以分成以下三种类型:
按驱动力性质分:有电动型和助力型两种。
按整车结构分:有两轮、三轮、电动轮椅三种。
按电动机形式及驱动方式分:有直驱车轮的轮毂式直流电动机、中轴链式驱动的柱式直流电动机、电动箱式驱动的柱式和盘式直流电动机。
1.2.3 电机
由于电动自行车要求的功率较小,所带有的能源有限,故希望电机的驱动系统的体积小、功率高,而其中以直流电机最为合适。目前,电动自行车所使用的电机大多数为有刷直流电动机,但是随着技术的进步与电子元件成本的不断下降,无刷直流电机将成为今后电动自行车的发展主流。
1、有刷直流电机
电动自行车上使用的有刷直流电机主要有两种:一是印制绕组电机,二是绕线盘式电机。前者的应用较后者多一点。有刷直流电机的优点是控制系统简单,成本较低,过载能力强,但需要换向器(整流子),影响电机的效率。
有刷直流电机又可以分为有齿和无齿两种。有刷有齿电机是一种高速电机,所谓“有齿”就是通过齿轮减速机构,将电机转速降低(国家标准规定电动自行车的转速不超过20)。由于高速电机通过齿轮减速,其特点是骑车者的感觉动力较强,而且爬坡的能力较强。但是电动轮毂是封闭的,只是在出厂前加注了润滑脂,用户很难进行日常保养。而且齿轮本身就有机械磨损,一年左右因为润滑不足导致齿轮磨损加剧,噪音增大,影响电机和电池的寿命。另一种是有刷无齿电机,这是一种外转子式电动机,没有齿轮减速机构,直接采用低速输出,消除了齿轮噪音,其电机输出转矩较有刷有齿电机要小,故动力性能和爬坡性能相对要弱一些。但运行安全,电刷磨损小、噪音小这是他的优点。
2、无刷直流电机
无刷直流电机由于没有电刷,不需要齿轮减速,从根本上消除了电刷磨损和齿轮磨损,不存在定期更换电刷,而且噪音很小。因此,他无干扰、寿命长、效率高、运行可靠、维护简单,且转速由于不受机械换向的限制,可在宽广的范围内平滑调速,与有刷直流电机相比成本低,但是控制系统复杂成本高。
无刷直流电机就起结构而言,是由电动机本体转子位置传感器以及电子电路换向组成。电动自行车上采用的是无刷电动轮毂电动机一般制成多相,如采用三相,它的电枢放在定子上,永磁磁极位于转子上。反映电动机定转子位置传感器输出信号,通过定子换向电路去驱动与电枢绕组联接的相应的功率器件,使电枢绕组依次馈电,从而在定子上产生一个跳跃式旋转磁场,拖动永磁转子旋转。随着转子位置的旋转,位置传感器通过电子换向线路不断的送出信号,以改变电枢绕组的通电状态,保证在一定的范围内定子磁场与转子磁场成正交关系,保持转矩连续不断的产生,输出机械功率,从而实现了无接触式电磁换向。
我国电动自行车用无刷直流电机的研制和生产厂家有上海微电机研究所、浙江卧龙集团等。今后二合一轮毂式无刷直流电机和无位置传感器无刷直流电机是电动自行车车用电机的发展方向。
1.2.4 电池
电池是电动车的动力源,电池质量直接影响电动自行车的行驶里程和寿命。目前电池选用的是铅酸电池、镍镉电池、镍氢电池等。
镍镉电池属碱性电池,我国产量较大,主要生产厂家有深圳比迪亚有限公司和上海电池厂等。镍镉电池可以快速充电,其过充、过放电性能均好,循环寿命较长,达2000次。但它的价格是铅酸电池的4.5倍,而且旧电池得不到很好的回收,会因重金属镉造成严重的污染。常用的是24V 5Ah,重量,一次充电续行里程,按标准的80%放电,寿命约500次。
1.2.5 控制器
 川公网安备: 51019002004831号
川公网安备: 51019002004831号