煤车自动锚定机械臂机电液系统综合设计(全套含CAD图纸)
收藏
资源目录

压缩包内文档预览:(预览前20页/共21页)
编号:10200860
类型:共享资源
大小:3.01MB
格式:ZIP
上传时间:2018-06-15
上传人:机****料
认证信息
个人认证
高**(实名认证)
河南
IP属地:河南
50
积分
- 关 键 词:
-
煤车
自动
锚定
机械
机电
电机
系统
综合
设计
全套
cad
图纸
- 资源描述:
-
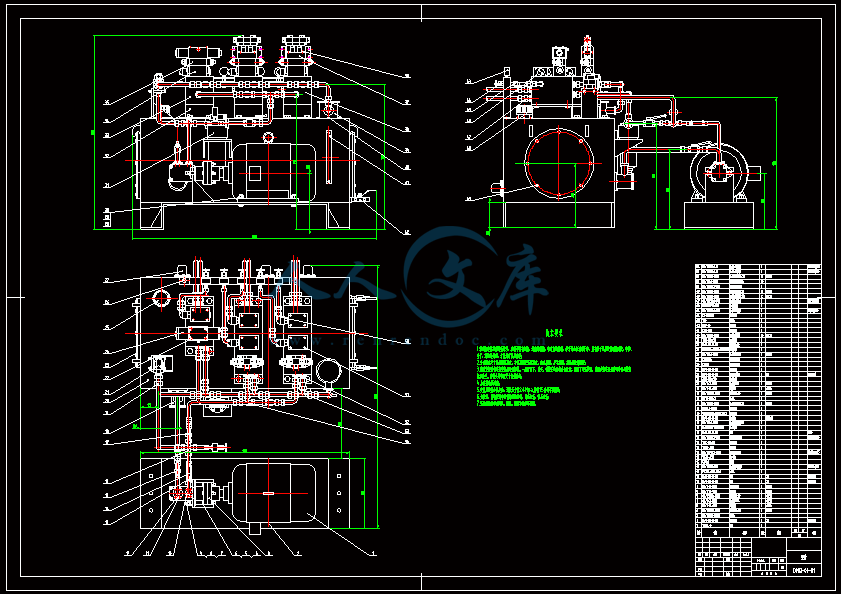
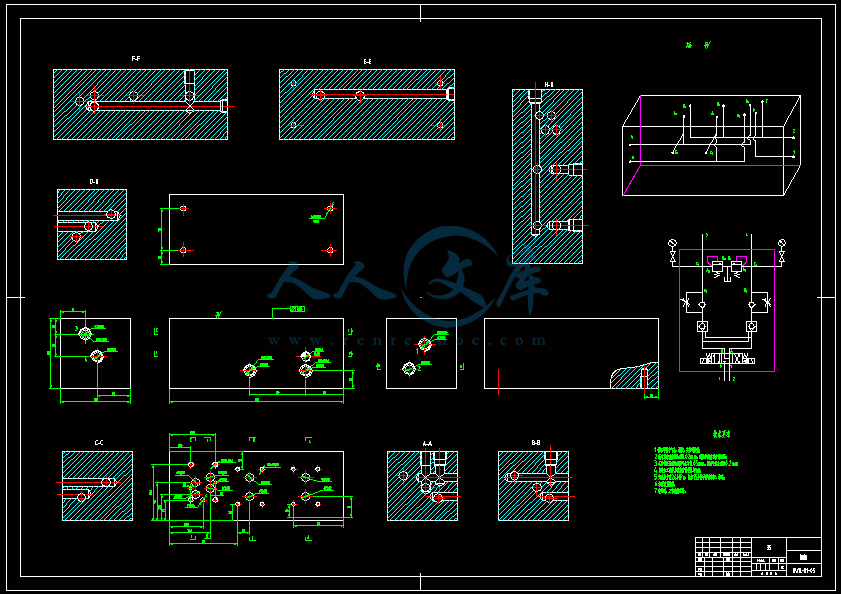

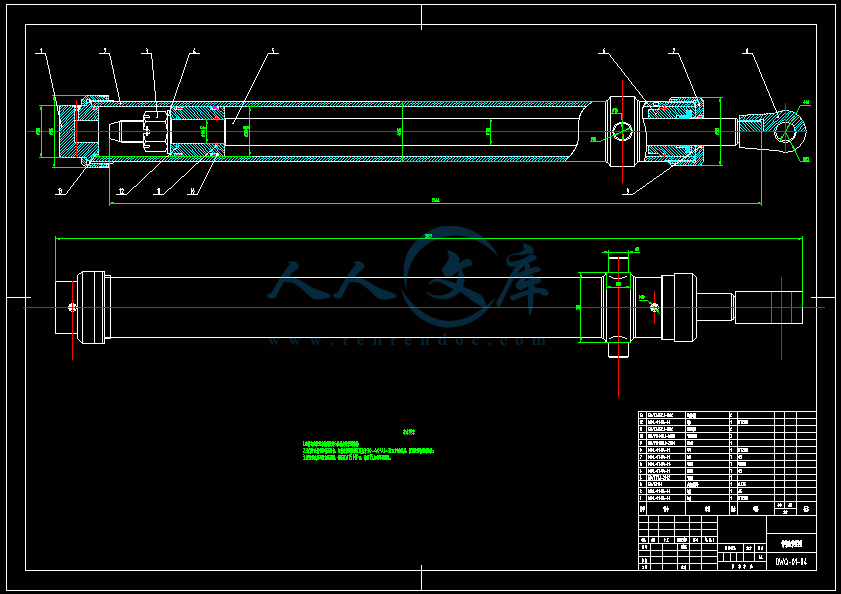



- 内容简介:
-
外文原文:Theory of fluid propertiesWe will concentrate mainly on three fluid properties in this chapter: The density which leads to mass and hence to hydraulic inertia effects. The viscosity which leads to the hydraulic friction effects. The compressibility and thus the bulk modulus which leads to the hydraulic system stiffness. Notice that the compressibility effect can be modified by air release, cavitation phenomena and by expansion of a pipe, hose or chamber containing the hydraulic fluid.1 Density and compressibility coefficient The density is the mass of a substance per unit volume:Density has dimensions of M/L and is expressed in kilograms per cubic meter kg/m. As mentioned previously the density is a function of the pressure and the temperature:This function can be approximated by the first three terms of a Taylor series:This can also be expressed as:WithAndThis equation is the linearized state equation for a liquid. Using the definition of the density, the two coefficients and B can also be expressed as:B is known as the isothermal bulk modulus or for simplicity the bulk modulus and is known as the cubical expansion coefficient. Since fluid density varies with the applied pressure, this implies that a given mass of fluid submitted to a pressure change changes its volume. This phenomenon leads to the definition of the compressibility coefficient : where is expressed in units Pa (or m/N). Considering the relation for a closed hydraulic circuit the mass is constant, and hence: it follows that Using the definition of the compressibility coefficient we obtain: More usually we use the bulk modulus B also known as the volumetric elasticity modulus: The relation between and B implies mass conservation. This relation must be RIGOROUSLY RESPECTED in the calculations. In the modeling and simulation context of fluid energy systems, disregarding the relation between and B leads to abnormal evolutions of pressure in the closed circuit submitted to compression and expansion cycles. This phenomenon is strongly accentuated if aeration occurs in the circuit (when dissolved air in the fluid reappears in the form of bubbles). We shall approach this point by examining the phenomena of aeration and cavitation. The air can also have adverse consequences on a fluid compressibility. In liquid air can be present in two forms: entrapped and dissolved. Entrapped air When the return pipe is not submersed in the tank the liquid jet can entrain some air bubbles in the tank. Another phenomenon that affects the quantity of air in liquid is the leakage. Figure 1: Liquid leakageFigure 2: Air is entrainedThis air stays in the liquid as cavities and can modify the fluid compressibility. In this context we talk about effective bulk modulus. Figure 3 shows the bulk modulus of a diesel fuel at 40 C with 0, 0.01, 0.1, 1, 10% air. The plot is obtained using the system shown. The model of the diesel fuel properties is based on accurate ex-perimental measurements and are designed for use with injection system which are very fast acting. For this reason air is assumed to be entrained rather than dissolved.Figure 3Dissolved airAir can also be dissolved in a liquid. A certain amount of air molecule can be part of the liquid. In this case the dissolved air does not significantly change the fluid properties. 2 Air release and cavitationAir can be dissolved or entrained in liquids and it is possible for air to change from one of these two forms to the other depending on the conditions to which the fluid is subjected. Suppose the fluid is in equilibrium with a certain percentage of dissolved gas (usually air: nitrogen and oxygen). Lowering the pressure above a critical value called the saturation pressure induces aeration. This is the process where the dissolved gas forms air bubbles in the liquid until all the dissolved gases or air are free. The exact point where all the dissolved gas has come out of solution is difficult to pin-point because it depends on the chemical composition and behavior of the gas. This is a non-symmetrical dynamic process: the growing process does not have the same dynamics as when air bubbles disappear. In consequence the total amount of bubbles created when the pressure drops may or may not be redissolved in the liquid when it rises again. If the pressure is dropped further and above another critical value called the vapor pressure, the fluid itself starts to vaporize. It corresponds to a liquid phase change. At some point only fluid vapor and gas exist. In liquid systems the term cavitation usually refers to the formation and collapse of cavities in the liquid even if cavities contain air or liquid vapor. To summarize with a sketch what we have introduced see above:Figure 4: Air release and cavitation The development of a cavity is now recognized as being associated with a nucleation center such as microscopic gas particles, wear or wall asperities. When the liquid is subjected to a tensile stress, cavities do not form as a result of liquid rupture but are caused by the rapid growth of these nuclei. To understand this, think of beer (or champagne if you prefer) in a bottle, when it is closed you see no air bubbles and the liquid does not look fizzy. The pressure in the bottle is above the saturation pressure of the gas in the liquid. When you open the bottle suddenly bubbles appear and so the dissolved gas (molecules of gas held in the liquid) starts to appear as gas.In fact the liquid is gas saturated and the atmospheric pressure is less than the saturation pressure of the liquid. This phenomenon is clearly not cavitation but air release (aeration). Considering nuclei effects, bubbles form only at particular places in your glass: around the glass (due to small asperities) and round any particles present in the liquid. Theoretically, if your liquid was perfectly pure and the wall of the system perfectly regular, air release or cavitation would occur with great difficulty! The key point about cavitation is that it is a phase change: the liquid changes to vapor. A comparison can be made between cavitation and boiling. If we look at the phase diagram below: Figure 5: Cavitation and boilingBoiling is a phase change at constant pressure and variable temperature and cavitation is a phase change at constant temperature and variable pressure. In any system air release starts first and if the pressure decreases further, cavitation may occur. This means that, sometimes, people talk about cavitation when the real phenomenon is air release. Both phenomena can lead to destruction of the material or component. In both cases it is entrained gas that causes the troubles. When cavities encounter high pressure in the downstream circuit, these bubbles or cavities can be unstable and can collapse implosively. The pressure developed at collapse can be large enough to cause severe mechanical damage in the containing vessel. It is well-known that hydraulic pumps and pipework can be badly damaged by cavitaton and air release.In all classical hydraulic systems air release and cavitation must be avoided to prevent material destruction but sometimes it is required like for injection systems to prepare the spray formation. 3 Viscosity Viscosity is a measure of the resistance of the fluid to flow. This characteristic has both positive and negative effects on fluid power systems. A low viscosity leads to oil leaks in the dead zone formed between the mechanical parts in movement, and a high viscosity will lead to loss of pressure in hydraulic ducts. Viscosity is a characteristic of liquids and gases and is manifested in motion through internal damping. Viscosity results from an exchange of momentum by molecular diffusion between two layers of fluid with different velocities. In this sense, the viscosity is a fluid property and not a flow property. Figure 6: ViscosityFigure 6 shows the relation between shearing constraint and difference of flow velocity between two layers .The definition of viscosity was first given by Newton. Between two layers of distance dy, the exerted force between these two layers is given by: where U(y) is the velocity depending on the radial position y and dU/dy the velocity gradient. This proportionality expresses the notion of Newtonian fluid and allows the introduction of defined as the dynamic viscosity or the absolute viscosity. The dimension of is MLT and the SI unit is kg/m/s or Pa s. The older unit is the Poise, P, which is 0.1 kg/m/s. However, this is very small and hence the milli Poise, mP, is the common unit which is 10-4 kg/m/s. The dynamic viscosity is the constant of proportionality between a stress and the intensity of shearing between two neighboring layers: However the absolute viscosity is not very often used in fundamental equations. For example the dynamics of the elementary volume between the two layers is expressed as:and thus using the shear stress calculation: In other formulas (e.g. Navier Stokes) the ratio between the absolute viscosity and the density occurs so often that a new parameter called the kinematic viscosity is introduced .of dimension L T and so the SI unit is the m/s. The older unit of kinematic viscosity is the Stoke, St, which is 10 m/s. However, even this is a very small unit and hence the centistoke cSt is the common unit with 1 cSt = 10 m/s. This parameter is easily measured with viscometers. Note that the viscosity varies significantly with the fluid temperature. Figure 7: Viscosity against temperatureNormally in absence of air release and cavitation the variation with pressure is not great unless the pressure is very extreme.Figure 8: Variation with pressureViscosity influence on the flow Another important aspect of the viscosity is its influence on the flow conditions of the fluid. We can distinguish two types of flow conditions: Laminar flow for which the flow lines are parallel and shearing forces create a pressure drop. Turbulent flow for which the fluid particles have a disordered, random movement leading to a loss of pressure. These two conditions can be distinguished using the Reynolds number which is defined as follows: WithU: average fluid velocity d: diameter of the duct (hydraulic diameter for others geometries): density : dynamic viscosity : kinematic viscosity The transition between laminar to turbulent flow occurs at the critical Reynolds number. This is not well defined, there exists always a transition region. In a hydraulic line, the critical Reynolds number is generally between 1500 to 2000. For uneven geometries (thin-walled orifices), the critical Reynolds number can be lower than 100. For non-circular cross sections, the hydraulic diameter can be used to determine the Reynolds number. Hydraulic diameter is defined as follows: We now give one example: Circular orifice of diameter: Flow through orifices Orifices (also called restrictions) can be fixed or variable and occur in huge numbers in fluid systems. Not surprisingly in Engineering courses a mathematical description is presented. This is usually based on Bernoullis equation and leads to the form where Cq is the flow coefficient. This is variously described as typically 0.7 or varying with orifice geometry and Reynolds number. The second alternative is obviously more correct. If we do take a constant value, we are forced to have the gradient of Q against infinity at the origin! This cannot be and if you try to implement it is a numerical disaster! Clearly the flow is laminar for sufficiently small pressure drops which means that Cq is certainly not constant. One solution is to perform detailed experiments and compute Cq against Reynolds number. In the context of the orifice (not necessarily circular) the Reynolds number is where U is a mean velocity and dh the hydraulic diameter. If we take U=Q/A, we end up with the form Cq =f(Q) and ultimately with It is possible to work with an implicit relationship like this but we would prefer an explicit formula.This is provided by introducing another dimensionless number known as the flow number and denoted by . This is defined as From a modeling point of view contains quantities we know. Using we have and provided we have ,we have an explicit relationship which is easy to evaluate. There are no more problems to obtain measurements forthan for and so the flow number form has many advantages. References : 1 McCloy D, Discharge Characteristics of Servo Valve Orifices, 1968 Fluid International Conference.2 R.C. Binder, “Fluid Mechanics”. 3rd Edition, 3rd Printing. Prentice-Hall, Inc., Englewood Cliffs,NJ. 1956.译文:液压油理论我们将在本章主要讨论液压油的三个特性:密度(使油液具有质量和液感效应);粘性(使油液具有液阻效应);可压缩性和体积弹性模量(使油液具有容性效应),值得提醒的是容性效应会受油液中析出的空气、气穴现象和装有油液的的管道、软管或油腔的影响。1密度和压缩系数密度是单位体积的物质的质量:密度的单位是千克每立方米kg/m。和先前提到的密度一样,这里的密度也是压力和温度的函数:这个函数可以大致用泰勒级的前三项来表示:这个式子也可表达成:同时并且这个方程是对液体线性化处理情况下的方程。利用对密度的定义,系数和B同样可以表达如下:B是通常大家所知的等温体积弹性模量或者对非专业人士来说体积弹性模量和就是在空间方向上的膨胀系数。既然液体的密度是随着作用的压力而变化的,那么这就意味着给定质量的液体在压力的变化下其体积也会发生相应的变化。这个现象导致压缩系数出现:这里的是用单位Pa 或者m/N表达的。考虑到对一个封闭的液压回路来说,其油液的质量是不变的,因此有:所以有利用压缩系数的定义我们可以得到:更为常见的是我们通常用体积弹性模量B(也常称作the volumetric elasticity modulus): 和 B的关系暗示着质量守恒,这个关系在计算中必须严格地遵守。在液压系统的建模和仿真过程中,忽视 和 B的关系将会导致闭环回路的压强的仿真不正常。如果掺气发生了的话,这个现象尤其应着重强调(当油液中溶解的空气以气泡的形式出现时),我们将通过检查掺气和气穴现象来解决这个问题。由于液体的可压缩性,空气也将会出现相反的结果。在液体中空气可以有两种存在的形式:以泡沫的形式存在于液体中和溶解于液体中。诱陷的空气当回油管没有完全淹在油箱中的油液里,液流将会带进一些气泡到油箱中去,另外一个导致一定数量的气进入液体中的现象的因素是泄漏。Figure 1: Liquid leakageFigure 2: Air is entrained这种以空穴的形式存留于液体中的空气可以改变液体的可压缩性。在这个段落我们谈论的是有效体积弹性模量。图3显示是溶有0, 0.01, 0.1, 1, 10%的空气的柴油在四十度的温度下的体积弹性模量值。这个曲线是通过该软件的系统自带的数据得到的。柴油的模型的性质是基于精确的测量实验的,并且这个实验是被设计用于高速动作的注射系统的。因此是假定带入的空气比溶入的多些。Figure 3溶解的空气空气也可以溶于液体中。一定量的空气分子能够成为液体的一部分。在这种情况下,溶解的空气不会对液体的性质产生多大的影响。2 空气的释放和气穴现象空气可以溶解或着存留于液体中并且空气可以由这两种形式转换成另一种依赖于液体的属性的形式。假定油液溶有一定百分数的气体(通常所说的空气:氮气和氧气)并处于平衡状态。降低油液的压力到低于其临界值(称作饱合压力值)将会导致掺气现象的产生。这就是溶解了的空气在液体中形成气泡直到所有溶解掉的气体或空气变得自由的过程这精确的临界值是难以确定的,因为这是取决于其化学组成和气体的行为。这是一个不对称的动态过程:这个渐进的过程不是和气泡消失时具有一样的动力学,因此当压力下降时产生的气泡在压力上升时可能不会被再次溶解掉。当压力再进一步下降并且临近另一个临界值(称作气化点)时,液体本身会开始气化。这是相应的液体过渡阶段。在某个压力值时仅仅只有气体和水蒸气存在。在流体界,术语气穴现象通常涉及到液体中空穴的形成与消亡,甚至空穴中有空气或蒸气存在。下面用一幅图来总结一下以上我们所介绍的:Figure 4: Air release and cavitation现在气穴的形成被认为与晶核点有关,比如精微的气体粒子、容器壁上的微刺。当液体受控于一个可变的压力时,空穴不会形成但会由于急速增长的晶核点导致气穴的产生。为了理解这个,试想一瓶啤酒(或者香槟),当它未被打开时,我们看不到气泡,并且液体看起来不是沸腾的。瓶子里的压力是高于液体中气体的饱合压力的。当你打开瓶子的时候,气泡就会迅速地出现并且溶解掉的气体(液体中的气体分子)开始以气体的形式出现。事实上液体就是饱合的气体,并且大气压是低于液体的饱合压力值的。这个现象明显不是气穴现象但是是气体释放现象。考虑到核子的影响,气泡仅仅形成于你的玻璃杯中特定的位置:在杯子的周围和任何液体中的粒子处。理论上讲,如果你的液体是绝对的纯净并且系统的壁子是绝对的光滑,那么气体释放和气穴现象是非常难产生的!气穴现象的关键点是它是一个液体转变为气体的过程。我们可以做一个之气穴现象和沸腾现象之间的区别实验。如果我们看到如下的图表的话:Figure 5: Cavitation and boiling沸腾就是一个在不变的压力和可变的温度下的相变过程,而气穴现象是一个在不变的温度和可变的压力下的相变过程。在任何系统中如果压力进一步下降,空气就会首选释放出来,这时气穴现象就有可能产生。这就意味着,有时人们所说的气穴现象就是气体释放现象。两种现象都会导致材料或元件的损坏。在这两种情况下带入的气体就会导致麻烦产生。当空穴在回路中遇到高压的情况时,气泡或者空穴就会不稳定,就会一下子消失。压力在崩溃的时候会足以在容器中导致严重的机械事故。大家都知道液压泵和管道工程会被气穴现象和气体释放严重破坏。在所有的传统的液压系统中,为了使材料免受损伤会采取方法避免气体释放和气穴现象的产生,但是有的时候在喷射系统中为了产生喷雾这些现象又是需要利用的。3 粘性粘性是用来衡量液流的阻尼性质的。这种特性对液压系统来说有好的一面也有消极的一面。一种低粘度的油液会在运动中机械部件中形成的闭死区导致泄漏,并且高粘度会导致
- 温馨提示:
1: 本站所有资源如无特殊说明,都需要本地电脑安装OFFICE2007和PDF阅读器。图纸软件为CAD,CAXA,PROE,UG,SolidWorks等.压缩文件请下载最新的WinRAR软件解压。
2: 本站的文档不包含任何第三方提供的附件图纸等,如果需要附件,请联系上传者。文件的所有权益归上传用户所有。
3.本站RAR压缩包中若带图纸,网页内容里面会有图纸预览,若没有图纸预览就没有图纸。
4. 未经权益所有人同意不得将文件中的内容挪作商业或盈利用途。
5. 人人文库网仅提供信息存储空间,仅对用户上传内容的表现方式做保护处理,对用户上传分享的文档内容本身不做任何修改或编辑,并不能对任何下载内容负责。
6. 下载文件中如有侵权或不适当内容,请与我们联系,我们立即纠正。
7. 本站不保证下载资源的准确性、安全性和完整性, 同时也不承担用户因使用这些下载资源对自己和他人造成任何形式的伤害或损失。

人人文库网所有资源均是用户自行上传分享,仅供网友学习交流,未经上传用户书面授权,请勿作他用。
 川公网安备: 51019002004831号
川公网安备: 51019002004831号